Plant Cell Wall-Like Soft Materials: Micro- and Nanoengineering, Properties, and Applications
- PMID: 39777633
- PMCID: PMC11711842
- DOI: 10.1007/s40820-024-01569-0
Plant Cell Wall-Like Soft Materials: Micro- and Nanoengineering, Properties, and Applications
Abstract
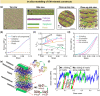
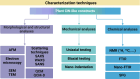
Plant cell wall (CW)-like soft materials, referred to as artificial CWs, are composites of assembled polymers containing micro-/nanoparticles or fibers/fibrils that are designed to mimic the composition, structure, and mechanics of plant CWs. CW-like materials have recently emerged to test hypotheses pertaining to the intricate structure-property relationships of native plant CWs or to fabricate functional materials. Here, research on plant CWs and CW-like materials is reviewed by distilling key studies on biomimetic composites primarily composed of plant polysaccharides, including cellulose, pectin, and hemicellulose, as well as organic polymers like lignin. Micro- and nanofabrication of plant CW-like composites, characterization techniques, and in silico studies are reviewed, with a brief overview of current and potential applications. Micro-/nanofabrication approaches include bacterial growth and impregnation, layer-by-layer assembly, film casting, 3-dimensional templating microcapsules, and particle coating. Various characterization techniques are necessary for the comprehensive mechanical, chemical, morphological, and structural analyses of plant CWs and CW-like materials. CW-like materials demonstrate versatility in real-life applications, including biomass conversion, pulp and paper, food science, construction, catalysis, and reaction engineering. This review seeks to facilitate the rational design and thorough characterization of plant CW-mimetic materials, with the goal of advancing the development of innovative soft materials and elucidating the complex structure-property relationships inherent in native CWs.
Keywords: Acellular wall; Biomimicry; Composites; Living materials; Soft matter; Synthetic plants.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: The authors declare no conflict of interest. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
A comprehensive review of the characterization of raw and surface-modified cellulosic plant fibers and their polyester- and epoxy-based composites.Int J Biol Macromol. 2025 Jul;318(Pt 4):145310. doi: 10.1016/j.ijbiomac.2025.145310. Epub 2025 Jun 16. Int J Biol Macromol. 2025. PMID: 40533003 Review.
-
Consumer Wearable Usage to Collect Health Data Among Adults Living in Germany: Nationwide Observational Survey Study.JMIR Mhealth Uhealth. 2025 Jun 11;13:e59199. doi: 10.2196/59199. JMIR Mhealth Uhealth. 2025. PMID: 40499135 Free PMC article.
-
Comparison of cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease.Cochrane Database Syst Rev. 2001;(3):CD003234. doi: 10.1002/14651858.CD003234. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003234. doi: 10.1002/14651858.CD003234.pub2. PMID: 11687058 Updated.
-
Biomimetic Three-Dimensional (3D) Scaffolds from Sustainable Biomaterials: Innovative Green Medicine Approach to Bone Regeneration.J Funct Biomater. 2025 Jun 29;16(7):238. doi: 10.3390/jfb16070238. J Funct Biomater. 2025. PMID: 40710453 Free PMC article. Review.
-
Modification Strategies of Kapok Fiber Composites and Its Application in the Adsorption of Heavy Metal Ions and Dyes from Aqueous Solutions: A Systematic Review.Int J Environ Res Public Health. 2022 Feb 25;19(5):2703. doi: 10.3390/ijerph19052703. Int J Environ Res Public Health. 2022. PMID: 35270400 Free PMC article.
Cited by
-
The Overexpression of ORR3 Negatively Regulates the Growth of Young Rice Roots by Reducing the Cell Size and the Number in the Root Meristematic Zone.Plants (Basel). 2025 May 27;14(11):1627. doi: 10.3390/plants14111627. Plants (Basel). 2025. PMID: 40508302 Free PMC article.
-
Three-dimensional Patterning Super-Black Silica-Based Nanocomposite Aerogels.Nanomicro Lett. 2025 Aug 20;18(1):36. doi: 10.1007/s40820-025-01870-6. Nanomicro Lett. 2025. PMID: 40833714 Free PMC article.
-
Exploring Plant Agro-Industrial By-Products as a Source of Fibrous Food Ingredients: A Review of Extraction Methods and Technological Properties.J Food Sci. 2025 Jul;90(7):e70408. doi: 10.1111/1750-3841.70408. J Food Sci. 2025. PMID: 40650498 Free PMC article. Review.
References
-
- N. Carpita, M. Tierney, M. Campbell, Molecular biology of the plant cell wall: searching for the genes that define structure, architecture and dynamics. Plant Mol. Biol. 47, 1–5 (2001). 10.1007/978-94-010-0668-2_1 - PubMed
-
- D.J. Cosgrove, Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 6, 850–861 (2005). 10.1038/nrm1746 - PubMed
-
- E.R. Lampugnani, G.A. Khan, M. Somssich, S. Persson, Building a plant cell wall at a glance. J. Cell Sci. 131, jcs207373 (2018). 10.1242/jcs.207373 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
