Electrophysiological Studies in Combination With Interim-Positron Emission Tomography Scan for Prevention of Severe Brentuximab-Vedotin-Induced Neurotoxicity
- PMID: 39777790
- PMCID: PMC11976687
- DOI: 10.1111/ejh.14384
Electrophysiological Studies in Combination With Interim-Positron Emission Tomography Scan for Prevention of Severe Brentuximab-Vedotin-Induced Neurotoxicity
Abstract
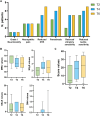
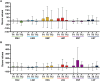
Brentuximab-vedotin (BV)-induced neurotoxicity (BVIN), a frequent adverse event caused by this monoclonal antibody, is the primary reason for dose modification or drug discontinuation, and is characterized by sensory, motor, and/or autonomic peripheral nerve dysfunctions. Although reversible, BVIN can persist for months or years after treatment and negatively affect quality of life (QoL). Currently, BVIN is managed by dose adjustment or drug interruption, leading to an increased risk of disease relapse. Therefore, early recognition and appropriate management are essential to improve clinical outcomes. In this real-life study, we identified predictive factors for moderate/severe BVIN to reduce the risk of irreversible neuropathy. A total of 22 patients treated with BV were enrolled and BVIN was monitored by electro-neurography and neurological examinations every 2 cycles of therapy, while QoL by clinical questionnaires. We showed that recovery rate from moderate/severe BVIN was low, and sensory nerves were the most affected, negatively impacting QoL. BV dose reduction based on interim PET re-evaluation in patients with hematological response resulted in a significant reduction of BVIN onset with high long-term QoL. Therefore, electrophysiological tests could be useful tools to prevent moderate/severe BVIN onset, and their combination with interim PET imaging could allow dosage adjustments thus simultaneously minimizing risks of disease relapse and BVIN development. However, further studies on larger prospective randomized cohorts are needed to confirm our preliminary results.
Keywords: brentuximab‐vedotin; lymphoma; neurotoxicity; prevention; quality of life.
© 2025 The Author(s). European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Brentuximab-Induced Peripheral Neurotoxicity: A Multidisciplinary Approach to Manage an Emerging Challenge in Hodgkin Lymphoma Therapy.Cancers (Basel). 2021 Dec 5;13(23):6125. doi: 10.3390/cancers13236125. Cancers (Basel). 2021. PMID: 34885234 Free PMC article. Review.
-
Brentuximab vedotin plus nivolumab as first-line therapy in older or chemotherapy-ineligible patients with Hodgkin lymphoma (ACCRU): a multicentre, single-arm, phase 2 trial.Lancet Haematol. 2020 Nov;7(11):e808-e815. doi: 10.1016/S2352-3026(20)30275-1. Epub 2020 Oct 1. Lancet Haematol. 2020. PMID: 33010817 Clinical Trial.
-
Rate and characteristics of inflammatory neuropathies associated with brentuximab vedotin therapy.Eur J Neurol. 2024 Jul;31(7):e16285. doi: 10.1111/ene.16285. Epub 2024 Mar 21. Eur J Neurol. 2024. PMID: 38511878 Free PMC article.
-
Real-world efficacy of brentuximab vedotin plus bendamustine as a bridge to autologous hematopoietic stem cell transplantation in primary refractory or relapsed classical Hodgkin lymphoma.Ann Hematol. 2020 Oct;99(10):2385-2392. doi: 10.1007/s00277-020-04204-1. Epub 2020 Aug 3. Ann Hematol. 2020. PMID: 32748163 Free PMC article.
-
Risk of adverse events in lymphoma patients treated with brentuximab vedotin: a systematic review and meta-analysis.Expert Opin Drug Saf. 2020 May;19(5):617-623. doi: 10.1080/14740338.2020.1718103. Epub 2020 Jan 24. Expert Opin Drug Saf. 2020. PMID: 31955620
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

