The European Drug-Drug Interaction (EuroDDI) Study Protocol: A Cross-Country Comparison of Drug-Drug Interaction Prevalence in the Older Community-Dwelling Population
- PMID: 39777812
- PMCID: PMC11706702
- DOI: 10.1002/pds.70092
The European Drug-Drug Interaction (EuroDDI) Study Protocol: A Cross-Country Comparison of Drug-Drug Interaction Prevalence in the Older Community-Dwelling Population
Abstract
Background: Drug-drug interactions (DDIs), highly prevalent amongst the elderly, can lead to avoidable medication-related harm. Cardiovascular and central nervous system (CNS) drugs are commonly implicated. To date, there is no consensus on how to measure DDIs, making comparisons across countries challenging.
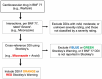
Objective: To (i) establish a common data model (CDM) to measure DDI prevalence in the older (aged ≥ 70 years) community-dwelling population of three European countries and (ii) compare and describe cardiovascular and CNS DDI prevalence rates across these countries.
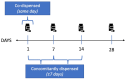
Methods: This cross-country study will apply a harmonised method of DDI identification and analysis using the WHO ATC classification system and national pharmacy claims data from three European countries (Ireland, Italy, Spain). Patients aged ≥ 70 years dispensed ≥ 2 medications during 2016 will be identified from each country's national database. 'Severe' cardiovascular and CNS DDIs (i.e., may result in a life-threatening event/permanent detrimental effect) will be identified using the British National Formulary and Stockley's Drug Interactions. Two separate lists of 'severe' DDIs, per medications reimbursed, will be applied to each database: (i) DDIs relevant to each individual country and (ii) DDIs relevant to all three countries. DDIs will be defined as co-dispensed (same day) and concomitantly (±7 days) dispensed.
Results: Descriptive statistics, including DDI prevalence and 95% confidence intervals, will be reported for each country. Prevalence will be pooled and compared across countries using random effects models and meta-regression, where feasible.
Conclusion: The EuroDDI study will develop a harmonised method to measure and compare DDI prevalence across health-related databases in Europe.
Keywords: adverse drug reactions; cardiovascular; common data model; drug interactions; elderly; medication safety; nervous system; pharmacoepidemiology; polypharmacy; protocol.
© 2025 The Author(s). Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Preston C. L., Stockley's Drug Interactions: A Source Book of Interactions, Their Mechanisms, Clinical Importance and Management, 12th ed. (London, UK: Pharmaceutical Press, 2019), 1.
-
- Magro L., Moretti U., and Leone R., “Epidemiology and Characteristics of Adverse Drug Reactions Caused by Drug–Drug Interactions,” Expert Opinion on Drug Safety 11, no. 1 (2012): 83–94. - PubMed
-
- Gurwitz J. H., Field T. S., Harrold L. R., et al., “Incidence and Preventability of Adverse Drug Events Among Older Persons in the Ambulatory Setting,” Journal of the American Medical Association 289, no. 9 (2003): 1107–1116. - PubMed
-
- Pedrós C., Formiga F., Corbella X., and Arnau J. M., “Adverse Drug Reactions Leading to Urgent Hospital Admission in an Elderly Population: Prevalence and Main Features,” European Journal of Clinical Pharmacology 72 (2016): 219–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GOIPG/2021/1213/Irish Research Council
- RL-15-1579/HRBI_/Health Research Board/Ireland
- SDAP-2021-020/HRBI_/Health Research Board/Ireland
- B01_23R/Government of Aragon
- RD21/0016/0019/Carlos III Institute of Health, Ministry of Science and Innovation (Spain), through the Network for Research on Chronicity, Primary Care, and Health Promotion (RICAPPS) awarded on the call for the creation of Health Outcomes-Oriented Cooperative Research Networks
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

