Ethylene increases the NaHCO3 stress tolerance of grapevines partially via the VvERF1B-VvMYC2-VvPMA10 pathway
- PMID: 39777954
- PMCID: PMC11933843
- DOI: 10.1111/pbi.14565
Ethylene increases the NaHCO3 stress tolerance of grapevines partially via the VvERF1B-VvMYC2-VvPMA10 pathway
Abstract
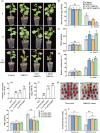
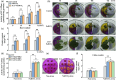
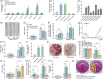
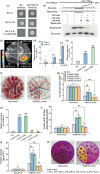
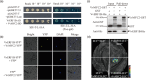
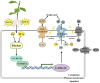
Here, we evaluated the role of ethylene in regulating the NaHCO3 stress tolerance of grapevines and clarified the mechanism by which VvERF1B regulates the response to NaHCO3 stress. The exogenous application of ACC and VvACS3 overexpression in grapevines and grape calli revealed that ethylene increased NaHCO3 stress tolerance, and this was accompanied by increased plasma membrane H+-ATPase (PMA) activity. The expression of VvERF1B was strongly induced by ACC, and overexpression of this gene in grapevines conferred increased NaHCO3 stress tolerance and enhanced PMA activity and H+ and oxalate secretion. Additionally, the function of VvERF1B was also verified using mutant transgenic grape calli and overexpression in Arabidopsis plants. The expression of VvPMA10 was strongly induced following the overexpression of VvERF1B in grapevine roots, and VvPMA10 was shown to regulate PMA activity, oxalate and H+ secretion, and NaHCO3 stress tolerance via its overexpression and mutation in grapevine roots, calli, and/or Arabidopsis. However, VvPMA10 was not a direct target gene of VvERF1B but was directly transactivated by VvMYC2. The function of VvMYC2 was shown to be similar to that of VvPMA10 via its overexpression and mutation in grape calli. Additional experiments revealed that the interaction of VvERF1B with VvMYC2 increased its ability to activate VvPMA10 expression and that VvMYC2 played a role in the VvERF1B-mediated pathway. Overall, the VvERF1B-VvMYC2-VvPMA pathway played a role in regulating ethylene-induced NaHCO3 stress tolerance in grapevines, and this process contributed to increases in PMA activity and H+ and oxalate secretion.
Keywords: NaHCO3 tolerance; VvERF1B; VvMYC2; VvPMA10; ethylene.
© 2025 The Author(s). Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships.
Figures


References
-
- Alhendawi, R.A. , Römheld, V. , Kirkby, E.A. and Marschner, H. (1997) Influence of increasing bicarbonate concentrations on plant growth, organic acid accumulation in roots and iron uptake by barley, sorghum, and maize. J. Plant Nutr. 20, 1731–1753.
-
- Chen, J. , Li, X. , Ye, X. , Guo, P. , Hu, Z. , Qi, G. , Cui, F. et al. (2021) An S‐ribonuclease binding protein EBS1 and brassinolide signaling are specifically required for Arabidopsis tolerance to bicarbonate. J. Exp. Bot. 72, 1449–1459. - PubMed
-
- Chen, Y. , Feng, P. , Tang, B. , Hu, Z. , Xie, Q. , Zhou, S. and Chen, G. (2022) The AP2/ERF transcription factor SlERF.F5 functions in leaf senescence in tomato. Plant Cell Rep. 41, 1181–1195. - PubMed
MeSH terms
Substances
Grants and funding
- 32072537/National Natural Science Foundation of China
- 31872068/National Natural Science Foundation of China
- SDAIT-06-03/Fruit Industrial Technology System of Shandong Province
- 2023TZXD015/Key Research and Development Program of Shandong Province
- 2022TZXD0011/Key Research and Development Program of Shandong Province
LinkOut - more resources
Full Text Sources
Other Literature Sources

