A Novel Lineage of Endosymbiotic Actinomycetales: Genome Reduction and Acquisition of New Functions in Bifidobacteriaceae Associated With Termite Gut Flagellates
- PMID: 39778056
- PMCID: PMC11707648
- DOI: 10.1111/1462-2920.70010
A Novel Lineage of Endosymbiotic Actinomycetales: Genome Reduction and Acquisition of New Functions in Bifidobacteriaceae Associated With Termite Gut Flagellates
Abstract
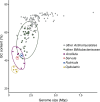
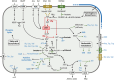
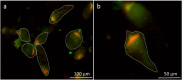
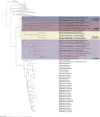
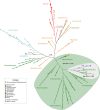
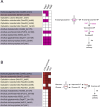
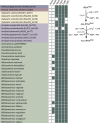
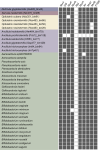
Cellulolytic flagellates are essential for the symbiotic digestion of lignocellulose in the gut of lower termites. Most species are associated with host-specific consortia of bacterial symbionts from various phyla. 16S rRNA-based diversity studies and taxon-specific fluorescence in situ hybridization revealed a termite-specific clade of Actinomycetales that colonise the cytoplasm of Trichonympha spp. and other gut flagellates, representing the only known case of intracellular Actinomycetota in protists. Comparative analysis of eleven metagenome-assembled genomes from lower termites allowed us to describe them as new genera of Bifidobacteriaceae. Like the previously investigated Candidatus Ancillula trichonymphae, they ferment sugars via the bifidobacterium shunt but, unlike their free-living relatives, experienced significant genome erosion. Additionally, they acquired new functions by horizontal gene transfer from other gut bacteria, including the capacity to produce hydrogen. Members of the genus Ancillula (average genome size 1.56 ± 0.2 Mbp) retained most pathways for the synthesis of amino acids, including a threonine/serine exporter, providing concrete evidence for the basis of the mutualistic relationship with their host. By contrast, Opitulatrix species (1.23 ± 0.1 Mbp) lost most of their biosynthetic capacities, indicating that an originally mutualistic symbiosis is on the decline.
Keywords: bifidobacteria; flagellates endosymbiosis; genome reduction; gut microbiota; horizontal gene transfer; termites.
© 2025 The Author(s). Environmental Microbiology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Biavati, B. 2015. “Bifidobacteriaceae.” In Bergey's Manual of Systematics of Archaea and Bacteria, 1–2. Ltd: John Wiley & Sons. 10.1002/9781118960608.fbm00008. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

