Genomic characterization and molecular predictive biomarkers for chemotherapy in patients with metastatic triple-negative breast cancer treated in a real-world setting
- PMID: 39778370
- PMCID: PMC11761257
- DOI: 10.1016/j.breast.2025.103874
Genomic characterization and molecular predictive biomarkers for chemotherapy in patients with metastatic triple-negative breast cancer treated in a real-world setting
Abstract
Purpose: We aimed to characterize genomic alterations with potential prognostic or predictive significance in patients with metastatic triple-negative breast cancer (mTNBC) treated with chemotherapy in a real-world setting.
Patients and methods: Next-generation sequencing with FoundationOne® CDx was conducted primarily on primary tumor tissue from 112 consecutive patients with mTNBC. Genomic alterations were subdivided into canonical oncogenic pathways and noted for their involvement in homologous recombination deficiency (HRD). Altered genes and pathways were correlated with overall survival (OS) and evaluated regarding their association with real-world progression-free survival (rwPFS) in patients treated with different chemotherapy agents. Occurrence of alterations were compared between patients with exceptional response and rapid progression to chemotherapy.
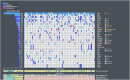
Results: After exclusion due to insufficient tumor tissue or clinical data, material from 97 patients was analyzed. The most frequently altered genes were TP53 (82 %), RAD21 (25 %) and PIK3CA (23 %). Altogether, 26 % of patients had an alteration leading to HRD. None of the analyzed alterations were associated with OS. Variants leading to HRD were associated with a prolonged rwPFS in patients treated with platinum-based chemotherapy in the first line setting (hazard ratio [HR], 0.31 [95 % CI: 0.12-0.84]). Exceptional responders more often exhibited alterations in the MYC and RAS/RTK pathways compared to rapid progressors.
Conclusions: Patients with tumor alterations in HRD-related genes seem to define subgroups that respond favorably to platinum-based chemotherapy. Further research into the genomic landscape of tumors from patients with rapid progression or exceptional response to different treatment strategies can provide insights into mechanisms of resistance and identify predictive biomarkers.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018 Nov 29;379(22):2108–2121. - PubMed
-
- Cortes J., Rugo H.S., Cescon D.W., Im S.A., Yusof M.M., Gallardo C., et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022 Jul 21;387(3):217–226. - PubMed
-
- Robson M., Im S.A., Senkus E., Xu B., Domchek S.M., Masuda N., et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017 Aug 10;377(6):523–533. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

