Modeling sacsin depletion in Danio Rerio offers new insight on retinal defects in ARSACS
- PMID: 39778749
- PMCID: PMC11757156
- DOI: 10.1016/j.nbd.2025.106793
Modeling sacsin depletion in Danio Rerio offers new insight on retinal defects in ARSACS
Abstract
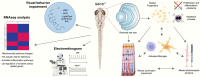
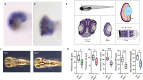
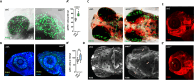
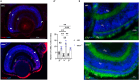
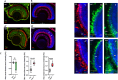
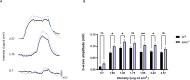
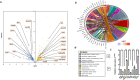
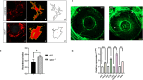
Biallelic mutations in the SACS gene, encoding sacsin, cause early-onset autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS), a neurodegenerative disease also characterized by unique and poorly understood retinal abnormalities. While two murine models replicate the phenotypic and neuronal features observed in patients, no retinal phenotype has been described so far. In a zebrafish knock-out strain that faithfully mirrors the main aspects of ARSACS, we observed impaired visual function due to photoreceptor degeneration, likely caused by cell cycle defects in progenitor cells. RNA-seq analysis in embryos revealed dysfunction in proteins related to fat-soluble vitamins (e.g., TTPA, RDH5, VKORC) and suggested a key role of neuroinflammation in driving the retinal defects. Our findings indicate that studying retinal pathology in ARSACS could be crucial for understanding the impact of sacsin depletion and may offer insights into halting disease progression.
Keywords: ARSACS; Neurological disorder; Retina development; Retinal abnormalities; Zebrafish.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

