Modulation of Mitochondria-Endoplasmic Reticulum Contacts (MERCs) by Small Molecules as a New Strategy for Restoring Lipid Metabolism in an Amyotrophic Lateral Sclerosis Model
- PMID: 39778888
- PMCID: PMC11770630
- DOI: 10.1021/acs.jmedchem.4c01368
Modulation of Mitochondria-Endoplasmic Reticulum Contacts (MERCs) by Small Molecules as a New Strategy for Restoring Lipid Metabolism in an Amyotrophic Lateral Sclerosis Model
Abstract
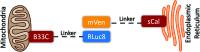
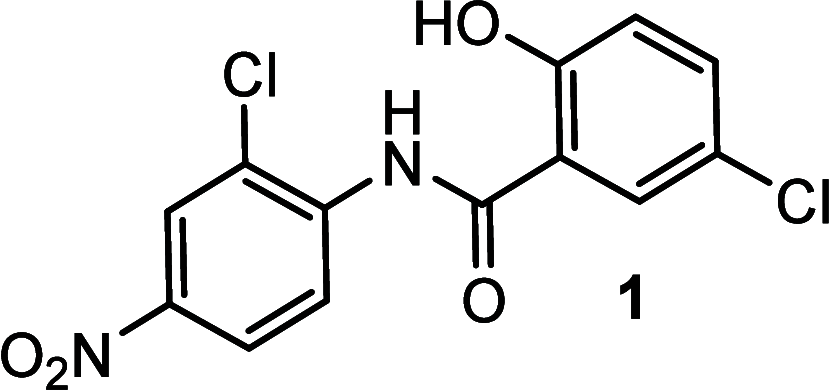
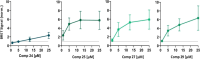
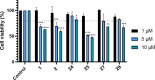
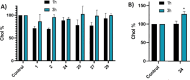
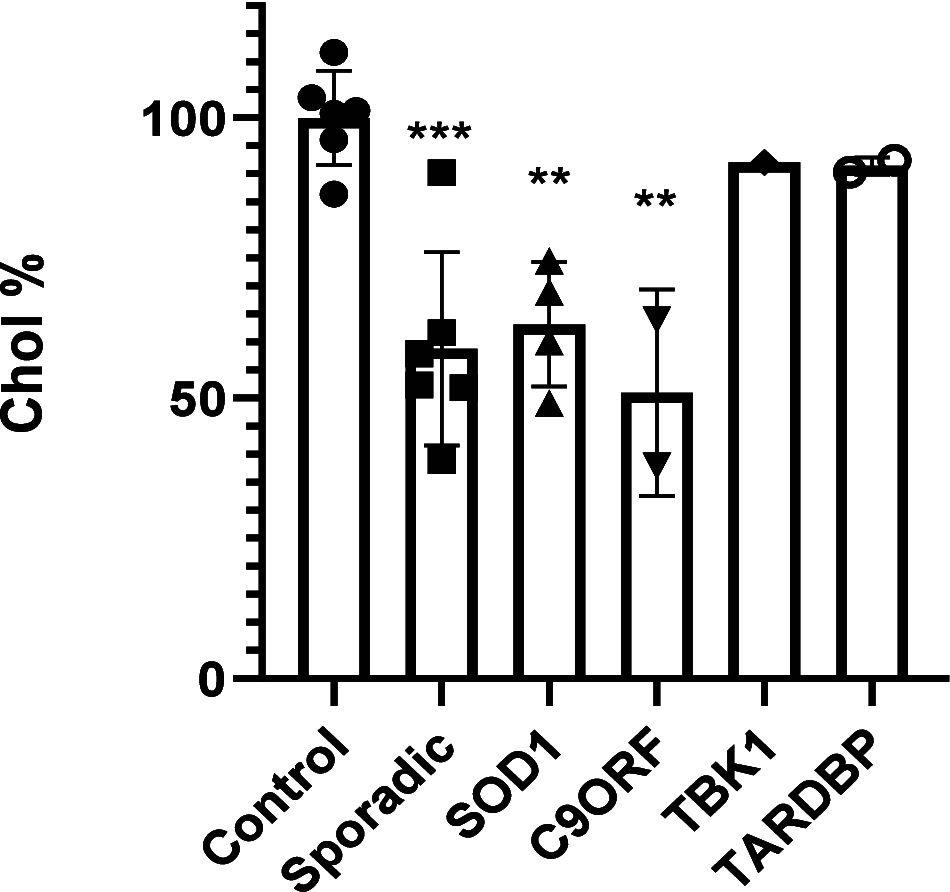
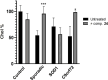
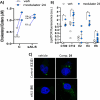
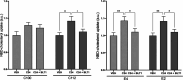
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease without effective treatment. The progressive motoneuron death in ALS is associated with alterations in lipid metabolism. As its regulation occurs in mitochondria-associated endoplasmic reticulum (ER) membranes (MAMs), modulation of mitochondria-ER contacts (MERCs) is emerging as a crucial factor in MAM formation and lipid metabolism control. Using the MERLIN biosensor in a high-throughput screening within the EU-OPENSCREEN ERIC, we discovered small molecules that increase MERCs in HCT116 cells, enhancing their ability to uptake cholesterol. We demonstrated that cholesterol trafficking is decreased in an ALS patient-derived cell model, and this trafficking is restored after treatment with the discovered MERC modulator 24. Electron microscopy revealed that treatment with compound 24 increases MERCs, promotes lipid droplet formation, and restores mitochondrial cristae. Overall, the brain-permeable MERC modulator, compound 24, may serve as a valuable pharmacological tool for studying MAM function and holds potential for in vivo studies in ALS and other MAM dysfunction diseases.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
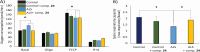



References
-
- Talbott E. O.; Malek A. M.; Lacomis D.. The epidemiology of amyotrophic lateral sclerosis. In Neuroepidemiology; Rosano C.; Ikram M. A.; Ganguli M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Vol. 138, pp 225–238. - PubMed
-
- Godoy-Corchuelo J. M.; Fernández-Beltrán L. C.; Ali Z.; Gil-Moreno M. J.; López-Carbonero J. I.; Guerrero-Sola A.; Larrad-Sainz A.; Matias-Guiu J.; Matias-Guiu J. A.; Cunningham T. J.; Corrochano S. Lipid metabolic alterations in the ALS-FTD spectrum of disorders. Biomedicines. 2022, 10, 1105.10.3390/biomedicines10051105. - DOI - PMC - PubMed
-
- Herrera-Cruz M. S.; Simmen T.. Over six decades of discovery and characterization of the architecture at mitochondria-associated membranes (MAMs). In Organelle Contact Sites: From Molecular Mechanism to Disease; Tagaya M.; Simmen T., Eds.; Springer: Switzerland, 2017; Vol. 997, pp 13–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

