Biologics in severe asthma: a state-of-the-art review
- PMID: 39778920
- PMCID: PMC11707604
- DOI: 10.1183/16000617.0088-2024
Biologics in severe asthma: a state-of-the-art review
Abstract
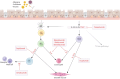
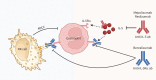
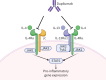
Asthma is considered severe if it remains uncontrolled despite optimal conventional therapy, characterised by poor symptom control, frequent exacerbations and increased exposure to systemic corticosteroids. This has a significant impact on morbidity, mortality and healthcare resource utilisation. Recent advances in the understanding of asthma heterogeneity and immunopathogenesis have helped delineate precise disease pathways. The discovery of these pivotal pathways has led to the development of highly effective biologic therapies. Currently available asthma biologics target immunoglobulin E, interleukin (IL)-5/IL-5Rα, IL-4Rα and thymic stromal lymphopoietin. Identification of specific asthma phenotypes, utilising easily measurable biomarkers, has paved the way towards personalised and precision asthma management. Biologic therapies play a significant role in reducing exacerbations, hospitalisations and the need for maintenance systemic steroids, while also improving the quality of life in patients with severe asthma. The evidence for their clinical efficacy comes from randomised controlled trials (RCTs), extension studies, metanalyses and real-world data. This review synthesises findings from early, pivotal RCTs and subsequent studies following the approval of biologics for severe asthma. The safety and efficacy data from these studies, completed in a variety of settings, provide practical perspectives on their application and enhance their generalisability.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: All authors report no conflicts of interest associated with the publication of this manuscript and there is no financial support for this work. Outside of this work: B. Gyawali has nothing to disclose. S.N. Georas reports research grants from NIH, consulting fees from AstraZeneca, ARS Pharma, Chiesi inc, Amarin Pharma and GH Research, payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Exchange CME, and acts as Chair of PreciSE Asthma Network steering committee (NIH/NHLBI) and Chair of DSMB for COPDGene study (NIH/NHLBI). S. Khurana reports research grants from the American Lung Association, royalties from Springer Nature, CME honoraria from CHEST/ACCP, European Respiratory Society, Healio, NYU, Cleveland Clinic, Thai Asthma Council, Eastern Pulmonary Conference, and serves as Regent-at-large for CHEST and Chair of DSMB for an NIH study.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
