Influenza vaccine outcomes: a meta-analysis revealing morbidity benefits amid low infection prevention
- PMID: 39778922
- PMCID: PMC11707602
- DOI: 10.1183/16000617.0144-2024
Influenza vaccine outcomes: a meta-analysis revealing morbidity benefits amid low infection prevention
Abstract
Background: The morbidity and mortality associated with influenza viruses are a significant public health challenge. Annual vaccination against circulating influenza strains reduces hospitalisations and increases survival rates but requires a yearly redesign of vaccines against prevalent subtypes. The complex genetics of influenza viruses with high antigenic drift create an ongoing challenge in vaccine development to address dynamic influenza epidemiology. Understanding the evolution of influenza viruses and the vaccine's effectiveness against different types and subtypes is pivotal to designing public health measures against influenza.
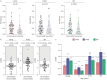
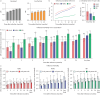
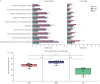
Methods: We conducted a systematic review and meta-analysis of 192 705 patients, collecting information on the incidence and severity of the disease. The results of this meta-analysis were further validated using data from 6 594 765 patients from TriNetX. We analysed the prevalence of the most common influenza A virus (IAV) subtypes (H1N1 and H3N2) and influenza B virus (IBV), as well as vaccination effectiveness against them in three age groups, given that age is associated with influenza disease severity.
Results: Our analysis reflects that overall vaccination against H1N1 IAV and IBV is effective in reducing infection and influenza-related complications in children aged <5 years old, individuals between 5 and 65 years old and older adults aged >65 years old. By contrast, while vaccination against H3N2 IAV is effective in protecting against infection in infants <5 years old, it provides reduced protection against infection in older individuals.
Conclusions: Despite higher infection rates, vaccination against H3N2 remains as highly effective as vaccination against H1N1 and IBV in reducing influenza-related morbidity and mortality in all age groups. Detailing vaccine effectiveness in terms of infection protection and disease burden across different age groups is necessary for understanding vaccine impacts in terms of other outcomes, e.g. hospitalisations, mortality and disease severity; for improving vaccine formulations and public awareness; and for enhancing vaccination campaigns to improve coverage and public acceptance.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: All authors have nothing to disclose.
Figures


Similar articles
-
Influenza Vaccine Effectiveness in Australia During 2017-2019.Influenza Other Respir Viruses. 2025 Jul;19(7):e70137. doi: 10.1111/irv.70137. Influenza Other Respir Viruses. 2025. PMID: 40669846 Free PMC article.
-
Binding antibody titers against the hemagglutinin and neuraminidase correlate with protection against medically attended influenza A and B disease.J Virol. 2025 Jun 17;99(6):e0039125. doi: 10.1128/jvi.00391-25. Epub 2025 May 13. J Virol. 2025. PMID: 40358209 Free PMC article.
-
Vaccines for preventing influenza in healthy adults.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD001269. doi: 10.1002/14651858.CD001269.pub6. Cochrane Database Syst Rev. 2018. PMID: 29388196 Free PMC article.
-
Real-world effectiveness of seasonal influenza vaccination and age as effect modifier: A systematic review, meta-analysis and meta-regression of test-negative design studies.Vaccine. 2024 Mar 19;42(8):1883-1891. doi: 10.1016/j.vaccine.2024.02.059. Epub 2024 Feb 28. Vaccine. 2024. PMID: 38423813
-
Influenza vaccines in immunosuppressed adults with cancer.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD008983. doi: 10.1002/14651858.CD008983.pub3. Cochrane Database Syst Rev. 2018. PMID: 29388675 Free PMC article.
Cited by
-
Unraveling Poland's unprecedented influenza surge in early 2025: increased viral severity or post-pandemic vulnerability?Pharmacol Rep. 2025 Aug;77(4):1134-1141. doi: 10.1007/s43440-025-00731-8. Epub 2025 Apr 24. Pharmacol Rep. 2025. PMID: 40268863
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
