Prospective Head-to-Head Comparison of 18F-PSMA PET/CT and 18F-NaF PET/CT for Assessing Bone Metastases in 160 Patients with Newly Diagnosed High-Risk Prostate Cancer
- PMID: 39778967
- PMCID: PMC11800739
- DOI: 10.2967/jnumed.124.268275
Prospective Head-to-Head Comparison of 18F-PSMA PET/CT and 18F-NaF PET/CT for Assessing Bone Metastases in 160 Patients with Newly Diagnosed High-Risk Prostate Cancer
Abstract
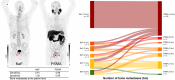
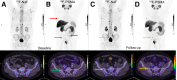
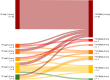
Prostate-specific membrane antigen (PSMA) PET/CT is increasingly used for primary staging in prostate cancer (PC), mainly because of its improved accuracy in detecting lymph node metastases compared with conventional imaging. However, the diagnostic benefit of PSMA PET/CT for detecting bone metastases is less well established. This study compares the diagnostic accuracy of 18F-PSMA PET/CT and 18F-NaF PET/CT for detecting bone metastases in patients newly diagnosed with PC. Methods: This prospective study included patients with histologically confirmed high-risk PC. All participants were referred from the department of urology to 18F-NaF PET/CT and underwent 18F-PSMA PET/CT within 3 weeks. Images were reviewed by 2 nuclear medicine physicians unaware of the results of the other imaging modality. Presence or absence of bone metastases and number of metastatic lesions were recorded. A reference standard was established at the patient level based on agreement between the 2 imaging modalities. In cases of concordance, both modalities were deemed correct. In cases of discordance, additional follow-up scans were performed. Diagnostic performance metrics, including sensitivity, specificity, and accuracy, were calculated. Results: In total, 160 participants were included. Sensitivity, specificity, and accuracy for detecting bone metastases at the patient level were 0.98, 0.99, and 0.99, respectively, for 18F-PSMA PET/CT, and 0.91, 1.00, and 0.97, respectively, for 18F-NaF PET/CT. No significant differences were found. The concordance rate of bone metastases between 18F-NaF and 18F-PSMA PET/CT at the patient level was observed in 154 patients (96.3%). 18F-PSMA PET/CT tended to identify more bone metastases per patient than 18F-NaF PET/CT. Conclusion: Both 18F-NaF and 18F-PSMA PET/CT exhibit high diagnostic accuracy for detecting bone metastases in newly diagnosed high-risk PC patients. 18F-PSMA PET/CT may detect additional metastatic lesions compared with 18F-NaF PET/CT. Subsequent 18F-NaF PET/CT may be redundant if no bone metastases are found on 18F-PSMA PET/CT.
Keywords: NaF PET/CT; PSMA PET/CT; bone metastases; diagnostic accuracy; prostate cancer.
© 2025 by the Society of Nuclear Medicine and Molecular Imaging.
Figures


References
-
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. - PubMed
-
- Schaeffer EM, Srinivas S, Adra N, et al. Prostate cancer, version 3.2024. J Natl Compr Canc Netw. 2024;22:140–150. - PubMed
-
- EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines on Prostate Cancer 2024. https://uroweb.org/guidelines/prostate-cancer. Accessed August 15, 2024.
-
- Hövels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–395. - PubMed
-
- Ghosh A, Heston WDW. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
