Cardiovascular events observed among patients in the etrasimod clinical programme: an integrated safety analysis of patients with moderately to severely active ulcerative colitis
- PMID: 39778975
- PMCID: PMC11748931
- DOI: 10.1136/bmjgast-2024-001516
Cardiovascular events observed among patients in the etrasimod clinical programme: an integrated safety analysis of patients with moderately to severely active ulcerative colitis
Abstract
Objective: Etrasimod is an oral, once-daily, selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC). S1P1 receptor expression on cardiac cells is involved in cardiac conduction. We report cardiovascular treatment-emergent adverse events (TEAEs) associated with S1P receptor modulators and other cardiovascular events in the etrasimod UC clinical programme.
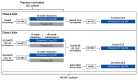
Methods: Patients were analysed in the Placebo-controlled UC cohort and All UC cohort. Incidence rates (IRs, per 100 patient-years) of cardiovascular-related TEAEs associated with S1P receptor modulators, including bradycardia/atrioventricular (AV) block and hypertension, and other cardiovascular events, including coronary artery disease (CAD) and cerebrovascular disease (CVD), were analysed.
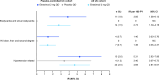
Results: In patients receiving etrasimod, cardiovascular-related TEAEs were infrequent (≤2.6% per AE). In the Placebo-controlled UC cohort, IRs (95% CIs) for cardiovascular-related TEAEs were higher for patients receiving etrasimod (n=577) vs placebo (n=314), respectively, for bradycardia/sinus bradycardia, 3.85 (1.58 to 6.13) vs 0 and AV block, 1.40 (0.03 to 2.76) vs 0; and numerically higher for hypertension, 5.31 (2.62 to 7.99) vs 3.40 (0.07 to 6.72). Most bradycardia/AV block events were reported on day 1. All bradycardia and hypertension TEAEs were non-serious. One serious second-degree AV block type 1 TEAE occurred in the etrasimod group; no events of second-degree AV block type 2 or higher were reported. One event each of CAD and CVD occurred in two patients receiving etrasimod.
Conclusions: In the etrasimod UC clinical programme, IRs of cardiovascular-related TEAEs and other cardiovascular events were low. Most cardiovascular-related TEAEs were non-serious.
Trial registration numbers: NCT02447302; NCT03945188; NCT03996369; NCT02536404; NCT03950232; NCT04176588.
Keywords: ADVERSE DRUG REACTIONS; CARDIOVASCULAR COMPLICATIONS; ULCERATIVE COLITIS.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SV has received lecture/speaker fees from AbbVie, Dr. Falk Pharma, Ferring Pharmaceuticals, Hospira, MSD, Takeda and Tillotts; has served as a consultant and advisory board member for AbbVie, AbolerIS Pharma, Alimentiv, Arena Pharmaceuticals, AstraZeneca, Avaxia, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, CVasThera, Dr. Falk Pharma, Eli Lilly, Ferring Pharmaceuticals, Galapagos, Genentech/Roche, Gilead Sciences, Hospira, Imidomics, Janssen Pharmaceuticals, Johnson and Johnson, Materia Prima, MiroBio, Morphic, MrMHealth, MSD, Mundipharma, Pfizer, Prodigest, Progenity, Prometheus Biosciences, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance Biopharma, Tillotts Pharma AG and Zealand Pharma; has received grant/research funding from AbbVie, Galapagos, MSD, Pfizer and Takeda. DTR has served as a consultant and advisory board member for AbbVie, Altrubio, Aslan Pharmaceuticals, Athos Therapeutics, Bellatrix Pharmaceuticals, Bristol Myers Squibb, Celgene, Chronicles, ClostraBio, Genentech/Roche, Gilead Sciences, Iterative Health, Janssen Pharmaceuticals, Lilly, Pfizer, Prometheus Biosciences, Reistone, Seres Therapeutics, Takeda, Target RWE and Trellus Health; has received grant/research funding from GastroIntestinal Research Foundation, Helmsley Charitable Trust and Takeda; has served on the board of trustees for Crohn’s & Colitis Foundation and Cornerstones Health; owns stock in Altrubio, Datos Health, and Iterative Health. LPB has received grant/research funding from Celltrion, Fresenius Kabi and Takeda; has served as a consultant for AbbVie, Abivax, Alimentiv, Alma Bio Therapeutics, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Biogen, Bristol Myers Squibb, Celltrion, CONNECT Biopharm, Cytoki Pharma, Eli Lilly, Enthera, Ferring Pharmaceuticals, Fresenius Kabi, Galapagos, Genentech, Gilead Sciences, Gossamer Bio, GSK, HAC-Pharma, IAG Image Analysis, Index Pharmaceuticals, Inotrem, Janssen Pharmaceuticals, Medac, Mopac, Morphic, MSD, Norgine, Novartis, OM Pharma, ONO Pharma, OSE Immunotherapeutics, Pandion Therapeutics, Par'Immune, Pfizer, Prometheus Biosciences, Protagonist, Roche, Sandoz, Takeda, Theravance Biopharma, Thermo Fisher, TiGenix, Tillotts, Viatris, Vifor and Ysopia; has received lecture/speaker fees from AbbVie, Amgen, Arena Pharmaceuticals, Biogen, Celltrion, Eli Lilly, Ferring Pharmaceuticals, Galapagos, Genentech, Gilead Sciences, Janssen Pharmaceuticals, Medac, MSD, Pfizer, Sandoz, Sanofi, Takeda, Tillotts, Viatris and Vifor; owns stock in CTMA. MCD has served as a consultant for AbbVie, Abivax, Arena Pharmaceuticals, AstraZeneca, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Genentech, Gilead Sciences, Janssen Pharmaceuticals, Pfizer, Prometheus Laboratories, Prometheus Biosciences, Takeda, and UCB; has received grant/research funding from Janssen; has shareholder/royalties in Trellus Health; has directorship/ownership interest in Trellus Health. MR has received unrestricted educational grants from AbbVie, Bristol Myers Squibb, Celgene, Genentech, Gilead Sciences, Janssen Pharmaceuticals, Pfizer, Takeda and UCB; has served as a consultant and advisory board member for AbbVie, Alfasigma S.p.A., Allergan, Amgen, Bristol Myers Squibb, Celgene, Genentech, Gilead Sciences, Lilly, Janssen Pharmaceuticals, Miraca Labs, Pfizer, Prometheus Biosciences, Salix, Seres, Takeda, TARGET Pharma Solutions and UCB; continuing medical education companies: CME Outfitters, Cornerstones, GI Health Foundation (GiHF), Imedex, MJH life sciences, and Remedy; has royalties in Wolters Kluwer Health. PMI has received grant support from Celltrion, Galapagos, MSD, and Takeda; has received lecture fees from AbbVie, Bristol Myers Squibb, Celgene, Celltrion, Dr. Falk Pharma, Ferring Pharmaceuticals, Galapagos, Gilead Sciences, Janssen Pharmaceuticals, MSD, Pfizer, Sandoz, Sapphire Medical, Shire, Takeda, Tillotts and Warner Chilcott; has served as an advisory board member for AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Genentech, Gilead Sciences, Hospira, Janssen Pharmaceuticals, Lilly, MSD, Pfizer, Pharmacosmos, Prometheus Biosciences, Roche, Samsung Bioepis, Sandoz, Takeda, Topivert, VH2, Vifor Pharma and Warner Chilcott. MG, KL, GG, JW, IM, AM, XG, JG and ABD are employees and shareholders of Pfizer. AJY has served as a consultant for AbbVie, Arena Pharmaceuticals, Bristol Myers Squibb, Pfizer and Takeda; has received lecture/speaker fees from Bristol Myers Squibb.
Figures
References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
