Amyloid-β-Driven Synaptic Deficits Are Mediated by Synaptic Removal of GluA3-Containing AMPA Receptors
- PMID: 39779375
- PMCID: PMC11867010
- DOI: 10.1523/JNEUROSCI.0393-24.2024
Amyloid-β-Driven Synaptic Deficits Are Mediated by Synaptic Removal of GluA3-Containing AMPA Receptors
Abstract
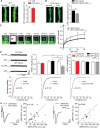
The detrimental effects of oligomeric amyloid-β (Aβ) on synapses are considered the leading cause for cognitive deficits in Alzheimer's disease. However, through which mechanism Aβ oligomers impair synaptic structure and function remains unknown. Here, we used electrophysiology and amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) imaging on mouse and rat neurons to demonstrate that GluA3 expression in neurons lacking GluA3 is sufficient to resensitize their synapses to the damaging effects of Aβ, indicating that GluA3-containing AMPARs at synapses are necessary and sufficient for Aβ to induce synaptic deficits. We found that Aβ oligomers trigger the endocytosis of GluA3 and promote its translocation toward endolysosomal compartments for degradation. Mechanistically, these Aβ-driven effects critically depend on the PDZ-binding motif of GluA3. A single point mutation in the GluA3 PDZ-binding motif prevented Aβ-driven effects and rendered synapses fully resistant to the effects of Aβ. Correspondingly, proteomics on synaptosome fractions from APP/PS1-transgenic mice revealed a selective reduction of GluA3 at an early age. These findings support a model where the endocytosis and lysosomal degradation of GluA3-containing AMPARs are a critical early step in the cascade of events through which Aβ accumulation causes a loss of synapses.
Keywords: AMPA; Alzheimer; GluA3; PDZ domain; amyloid; synapse.
Copyright © 2025 Reinders et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






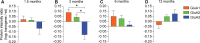
Similar articles
-
Amyloid-β effects on synapses and memory require AMPA receptor subunit GluA3.Proc Natl Acad Sci U S A. 2016 Oct 18;113(42):E6526-E6534. doi: 10.1073/pnas.1614249113. Epub 2016 Oct 5. Proc Natl Acad Sci U S A. 2016. PMID: 27708157 Free PMC article.
-
Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption.J Biol Chem. 2010 Mar 5;285(10):7619-32. doi: 10.1074/jbc.M109.057182. Epub 2009 Dec 23. J Biol Chem. 2010. PMID: 20032460 Free PMC article.
-
{beta}-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution.J Biol Chem. 2009 Apr 17;284(16):10639-49. doi: 10.1074/jbc.M806508200. Epub 2009 Feb 24. J Biol Chem. 2009. PMID: 19240035 Free PMC article.
-
Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease.Mol Neurodegener. 2014 Nov 14;9:48. doi: 10.1186/1750-1326-9-48. Mol Neurodegener. 2014. PMID: 25394486 Free PMC article. Review.
-
Ca2+-permeable AMPA receptor: A new perspective on amyloid-beta mediated pathophysiology of Alzheimer's disease.Neuropharmacology. 2017 Jan;112(Pt A):221-227. doi: 10.1016/j.neuropharm.2016.08.022. Epub 2016 Aug 22. Neuropharmacology. 2017. PMID: 27561971 Review.
Cited by
-
Ginsenoside C-K inhibits Aβ oligomer-induced Alzheimer's disease pathology progression by regulating microglia-neuron interactions.IBRO Neurosci Rep. 2025 May 21;18:783-793. doi: 10.1016/j.ibneur.2025.05.007. eCollection 2025 Jun. IBRO Neurosci Rep. 2025. PMID: 40510292 Free PMC article.
-
Early involvement of D-serine in β-amyloid-dependent pathophysiology.Cell Mol Life Sci. 2025 Apr 28;82(1):179. doi: 10.1007/s00018-025-05691-z. Cell Mol Life Sci. 2025. PMID: 40293541 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
