In vitro culture and confocal microscopy study of Maritrema gratiosum Nicoll, 1907 (Digenea): From metacercaria to ovigerous adult
- PMID: 39779536
- PMCID: PMC11711744
- DOI: 10.1007/s00436-024-08446-0
In vitro culture and confocal microscopy study of Maritrema gratiosum Nicoll, 1907 (Digenea): From metacercaria to ovigerous adult
Abstract
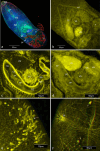
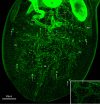
This study set out to characterise the in vitro development, including musculature, of the microphallid parasite of the barnacle Semibalanus balanoides (Linnaeus, 1767), Maritrema gratiosum Nicoll, 1907 collected in Scotland. An in vitro culture model was developed to obtain ovigerous adults of M. gratiosum and their morphology was observed. Different media were tested and NCTC-109 was chosen as the best medium. The effects of different concentrations of serum upon adult longevity, size and egg production was measured. Survival for 10-days was achieved when flukes were cultured in NCTC-109 plus chicken serum and antibiotics. Forty percent chicken serum seemed to provide better results in terms of survival time and producing flukes with the largest body lengths. Both normal and abnormal eggs were observed from adults cultured in vitro. Confocal microscopy was undertaken to provide details of the development of the parasite's ultrastructure, including musculature, during the course of in vitro culture. While the musculature of M. gratiosum was similar to that of other microphallids, some additional novel structures were observed, most notably a ligament connecting pars prostatica and seminal vesicle and a racket-shaped excretory bladder. This study has provided greater insight into the biology M. gratiosum, and also developed a good in vitro model which might be applied to ecological or medical research in the future.
Keywords: Barnacle parasites; Egg production; Excystment rates; Metacercarial development; Musculature; Spermatogenesis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: No ethical approval was required for the work with barnacles; however, our practices align with ethical principles regarding the collection and treatment of animals and adhere to the animal welfare regulations in the University of Stirling and the UK. Consent to participate: All authors consent to participate in this publication. Consent for publication: All authors consent to publish the manuscript. Competing interests: The authors declare no competing interests. Research data: Available on request by contacting the lead author. Clinical trial: Clinical trial number: not applicable.
Figures





References
-
- Abdulla MH, Ruelas DS, Wolff B, Snedecor J, Lim KC, Xu F, Renslo AR, Williams J, McKerrow JH, Caffrey CR (2009) Drug discovery for schistosomiasis: hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Negl Trop Dis 3(7):e478. 10.1371/journal.pntd.0000478 - PMC - PubMed
-
- Augot D, Rondelaud D, Dreyfuss G, Cabaret J (1997) Fasciola hepatica: in vitro production of daughter rediae and cercariae from first- and second-generation rediae. Parasitol Res 83:383–385 - PubMed
-
- Basch PF (1981a) Cultivation of Schistosoma mansoni in vitro. I. Establishment of cultures from cercariae and development until pairing. J Parasitol 67(2):179–185 - PubMed
-
- Basch PF (1981b) Cultivation of Schistosoma mansoni in vitro. II. Production of infertile eggs by worm pairs cultured from cercariae. J Parasitol 67(2):186–190 - PubMed
-
- Benjamin LR, James BL (1987) The development of the metacercariae of Maritrema linguilla Jäg., 1908 (Digenea: Microphallidae) in the intermediate host, Ligia oceanica (L.). Parasitology 94:221–231
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

