Experiences of patients with metastatic colorectal cancer participating in a supervised exercise intervention during chemotherapy
- PMID: 39779537
- PMCID: PMC11711133
- DOI: 10.1007/s00520-024-09101-1
Experiences of patients with metastatic colorectal cancer participating in a supervised exercise intervention during chemotherapy
Abstract
Purpose: Patients with metastatic colorectal cancer (mCRC) undergoing systemic treatment often experience toxicities. Although exercise may improve physical fitness and quality of life and counteract treatment toxicity, knowledge in patients with mCRC is limited. The ongoing randomized controlled AMICO trial evaluates the effects of supervised exercise on clinical outcomes. The present qualitative study was a pre-planned part of this trial aiming to capture adherence, satisfaction, and perceived effects of exercise among patients with mCRC.
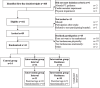
Methods: Patients with mCRC receiving first-line systemic treatment were randomized (1:1:1) to a control group or one of two supervised exercise arms including continuous aerobic exercise with either resistance exercises or high-intensity interval training. Semi-structured interviews with patients in the exercise arms were transcribed verbatim and thematically analyzed. Descriptive data on adherence (exercise logs) and satisfaction (questionnaire) was collected to complement and contextualize the qualitative findings.
Results: Twenty-one patients were interviewed. Median exercise attendance was 67% [IQR 35-91], and the median satisfaction score was 8 [IQR 8-9] out of 10. Patients valued the guidance and knowledge of the physical therapist and expressed interindividual preferences regarding training content. Patients experienced that exercise improved their physical and mental wellbeing and helped them to endure treatment. Perceived exercise barriers were treatment toxicity, physical problems, and hospital appointments. Perceived exercise facilitators included adequate tailoring and internal or external motivation.
Conclusion: Patients with mCRC appreciated exercise during systemic treatment and perceived several beneficial effects, both physically and mentally. Exercise attendance varied and barriers were mainly treatment- and disease-related.
Trial registration: Clinical trial.
Gov id: NCT04754672. Date of registration: 04-12-2020.
Keywords: Exercise; Physical activity; Qualitative study; Systemic treatment; Treatment tolerability.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Medical Ethics Committee of East Netherlands (METC2020-6867) and institutional review boards of all participating hospitals. Consent to participate: Informed consent was obtained from all individual participants included in the study. Competing interests: The authors declare no competing interests.
Figures
References
-
- Bray F et al (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74:2295 - PubMed
-
- Van Cutsem E et al (2014) Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii1-9 - PubMed
-
- Cremolini C et al (2020) Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol 38:JCO2001225 - PubMed
-
- Van Cutsem E et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27(8):1386–1422 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical