Low-temperature vacuum evaporation as a novel dehydration process for the long-term preservation of transplantable human corneal tissue
- PMID: 39779599
- PMCID: PMC11711135
- DOI: 10.1007/s10561-024-10155-y
Low-temperature vacuum evaporation as a novel dehydration process for the long-term preservation of transplantable human corneal tissue
Abstract
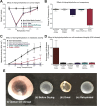
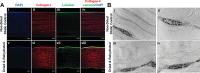
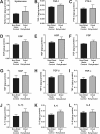
Globally there is a shortage of available donor corneas with only 1 cornea available for every 70 needed. A large limitation to corneal transplant surgery is access to quality donor tissue due to inadequate eye donation services and infrastructure in many countries, compounded by the fact that there are few available long-term storage solutions for effectively preserving spare donor corneas collected in countries with a surplus. In this study, we describe a novel technology termed low-temperature vacuum evaporation (LTVE) that can effectively dry-preserve surplus donor corneal tissue, allowing it to be stored for approximately 5 years, shipped at room temperature, and stored on hospital shelves before rehydration prior to ophthalmic surgery. The dry-preserved corneas demonstrate equivalent biological characteristics to non-dried donor tissue, with the exception that epithelial and endothelial cells are removed and keratocytes are rendered non-viable and encapsulated within the preserved extracellular matrix. Structure and composition of the dried and rehydrated corneas remained identical to that of non-dried control corneas. Matrix-bound cytokines and growth factors were not affected by the drying and rehydration of the corneas. The ability to preserve human donor corneas using LTVE will have considerable impact on global corneal supply; utilisation of preserved corneas in lamellar keratoplasties, corneal perforations, ulcers, and tectonic support, will allow non-preserved donor tissue to be reserved for where it is truly required.
Keywords: Cornea; Cryopreservation; Lyophilisation; Tissue processing; Transplant preservation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: OM, LB and LS declare they have no relevant financial or non-financial interests to disclose. AH declares a relationship with NuVision Biotherapies Ltd, Nottingham that includes employment and equity or stocks. EB declares a relationship with NuVision Biotherapies Ltd, Nottingham that includes employment. Ethical approval and consent to participate: Anonymised human corneas surplus to transplant requirement were obtained from SightLife (now CorneaGen, Seattle, WA, USA) under a materials transfer agreement. All work was performed in a laboratory under a research license from the UK Human Tissue Authority, UK. Informed consent was obtained from donors/relative prior to collection. Institutional ethical approval was not required as samples arrived anonymised, and consent was held at SightLife.
Figures

References
-
- Ang M, Moriyama A, Colby K, Sutton G, Liang L, Sharma N, Hjortdal J, Lam SC, D., P Williams, G., Armitage, J., & S Mehta, J. (2020) Corneal transplantation in the aftermath of the COVID-19 pandemic: an international perspective. Br J Ophthalmol 104(11):1477–1481. 10.1136/bjophthalmol-2020-317013 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

