CCR5-ligand decorated rilpivirine lipid-based nanoparticles for sustained antiretroviral responses
- PMID: 39779664
- PMCID: PMC11711180
- DOI: 10.1038/s41467-024-55544-9
CCR5-ligand decorated rilpivirine lipid-based nanoparticles for sustained antiretroviral responses
Abstract
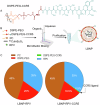
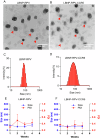
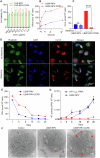
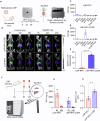
Antiretroviral therapy (ART) improves the quality of life for those living with the human immunodeficiency virus type one (HIV-1). However, poor compliance reduces ART effectiveness and leads to immune compromise, viral mutations, and disease co-morbidities. Here we develop a drug formulation in which a lipid-based nanoparticle (LBNP) carrying rilpivirine (RPV) is decorated with the C-C chemokine receptor type 5 (CCR5) targeting peptide. This facilitates extended drug persistence within myeloid cells. Particle delivery to viral reservoirs is tracked by positron emission tomography. The CCR5-mediated LBNP cell uptake and retention reduce HIV-1 replication in human monocyte-derived macrophages and infected humanized mice (hu mice). Focused ultrasound with microbubbles mediated blood brain barrier (BBB) disruption allows the CCR5-targeted LBNP to penetrate the BBB and reach brain myeloid cells. These findings offer a role for CCR5-targeted therapeutics in antiretroviral delivery to optimize HIV suppression.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare that Dr. Howard Gendelman co-founder of Exavir Therapeutics, Inc. The biotechnology company is developing ultra-long-acting drugs. The drugs in development are not linked to those created in the current report. All other authors declare no competing interests.
Figures


Update of
-
CCR5 Decorated Rilpivirine Lipid Nanoparticles Build Myeloid Drug Depots Which Sustains Antiretroviral Activities.Res Sq [Preprint]. 2024 Jun 3:rs.3.rs-4433306. doi: 10.21203/rs.3.rs-4433306/v1. Res Sq. 2024. Update in: Nat Commun. 2025 Jan 8;16(1):513. doi: 10.1038/s41467-024-55544-9. PMID: 38883780 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

