Organocatalytic enantioselective synthesis of double S-shaped quadruple helicene-like molecules
- PMID: 39779671
- PMCID: PMC11711666
- DOI: 10.1038/s41467-024-55590-3
Organocatalytic enantioselective synthesis of double S-shaped quadruple helicene-like molecules
Abstract
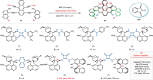
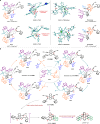
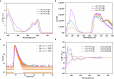
Helicene-shaped molecules are compelling chemical structures with unique twisted helical chirality and remarkable properties. Although progress occurs in the catalytic asymmetric synthesis of helicene (-like) molecules, the enantioselective synthesis of multiple helicenes, especially four or higher helicity, is still challenging and has yet to be achieved. Herein, we report an organocatalytic [4 + 2] cycloadditions to achieve double S-shaped quadruple helicene-like molecules with high enantioselectivity (up to 96% e.e.). The enantioselective synthesis of (P,P,P,P) and (M,M,M,M) configurational quadruple helical molecules can be achieved by modulating the structure of the catalyst. Density functional theory (DFT) calculations show that the reaction involves the formation of a duplex vinylidene ortho-quinone methide (VQM) intermediate and two successive cycloaddition reactions. Configurational stability studies elucidate the isomerization process between the isomers. In addition, the structural features and optical properties of the quadruple helicene-like molecules were investigated to explore their potential applications.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

