Three-dimensional architecture and linearized mapping of vibrissa follicle afferents
- PMID: 39779697
- PMCID: PMC11711312
- DOI: 10.1038/s41467-024-55468-4
Three-dimensional architecture and linearized mapping of vibrissa follicle afferents
Abstract
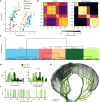
Understanding vibrissal transduction has advanced by serial sectioning and identified afferent recordings, but afferent mapping onto the complex, encapsulated follicle remains unclear. Here, we reveal male rat C2 vibrissa follicle innervation through synchrotron X-ray phase contrast tomograms. Morphological analysis identified 5% superficial, ~32 % unmyelinated and 63% myelinated deep vibrissal nerve axons. Myelinated afferents consist of each one third Merkel and club-like, and one sixth Ruffini-like and lanceolate endings. Unsupervised clustering of afferent properties aligns with classic morphological categories and revealed previously unrecognized club-like afferent subtypes distinct in axon diameter and Ranvier internode distance. Myelination and axon diameters indicate a proximal-to-distal axon-velocity gradient along the follicle. Axons innervate preferentially dorso-caudally to the vibrissa, presumably to sample contacts from vibrissa protraction. Afferents organize in axon-arms innervating discrete angular territories. The radial axon-arm arrangement around the vibrissa maps into a linear representation of axon-arm bands in the nerve. Such follicle linearization presumably instructs downstream linear brainstem barrelettes. Synchrotron imaging provides a synopsis of afferents and mechanotransductory machinery.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




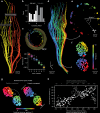
References
-
- Maderson, P. F. When? Why? and How? Some speculations on the evolution of the vertebrate integument. Am. Zool.12, 159–171 (1972). - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

