Autonomous ribosome biogenesis in vitro
- PMID: 39779722
- PMCID: PMC11711502
- DOI: 10.1038/s41467-025-55853-7
Autonomous ribosome biogenesis in vitro
Abstract
Ribosome biogenesis is pivotal in the self-replication of life. In Escherichia coli, three ribosomal RNAs and 54 ribosomal proteins are synthesized and subjected to cooperative hierarchical assembly facilitated by numerous accessory factors. Realizing ribosome biogenesis in vitro is a critical milestone for understanding the self-replication of life and creating artificial cells. Despite its importance, this goal has not yet been achieved owing to its complexity. In this study, we report the successful realization of ribosome biogenesis in vitro. Specifically, we developed a highly specific and sensitive reporter assay for the detection of nascent ribosomes. The reporter assay allowed for combinatorial and iterative exploration of reaction conditions for ribosome biogenesis, leading to the simultaneous, autonomous synthesis of both small and large subunits of ribosomes in vitro through transcription, translation, processing, and assembly in a single reaction space. Our achievement represents a crucial advancement toward revealing the fundamental principles underlying the self-replication of life and creating artificial cells.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Kyoto University have filed a patent application on in vitro ribosome biogenesis (by YK and WA). TS is employed at TechnoPro, Inc. These competing interests do not alter our adherence to the journal policies on sharing data and materials. The other authors declare no competing interests.
Figures


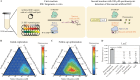
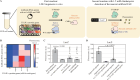
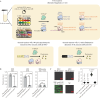
References
MeSH terms
Substances
Grants and funding
- 19K16109/MEXT | Japan Society for the Promotion of Science (JSPS)
- 26830139/MEXT | Japan Society for the Promotion of Science (JSPS)
- 23K18110/MEXT | Japan Society for the Promotion of Science (JSPS)
- 23K26466/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22J22251/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

