Biochemical and structural characterization of Rab3GAP reveals insights into Rab18 nucleotide exchange activity
- PMID: 39779760
- PMCID: PMC11711316
- DOI: 10.1038/s41467-025-55828-8
Biochemical and structural characterization of Rab3GAP reveals insights into Rab18 nucleotide exchange activity
Abstract
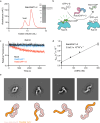
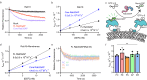
The heterodimeric Rab3GAP complex is a guanine nucleotide exchange factor (GEF) for the Rab18 GTPase that regulates lipid droplet metabolism, ER-to-Golgi trafficking, secretion, and autophagy. Why both subunits of Rab3GAP are required for Rab18 GEF activity and the molecular basis of how Rab3GAP engages and activates its cognate substrate are unknown. Here we show that human Rab3GAP is conformationally flexible and potentially autoinhibited by the C-terminal domain of its Rab3GAP2 subunit. Our high-resolution structure of the catalytic core of Rab3GAP, determined by cryo-EM, shows that the Rab3GAP2 N-terminal domain binds Rab3GAP1 via an extensive interface. AlphaFold3 modelling analysis together with targeted mutagenesis and in vitro activity assay reveal that Rab3GAP likely engages its substrate Rab18 through an interface away from the switch and interswitch regions. Lastly, we find that three Warburg Micro Syndrome-associated missense mutations do not affect the overall architecture of Rab3GAP but instead likely interfere with substrate binding.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.E.B. reports personal fees from Scorpion Therapeutics and Reactive Therapeutics and research contracts from Novartis and Calico Life Sciences. All other authors declare no competing interests.
Figures





References
-
- Derby, M. C. & Gleeson, P. A. New insights into membrane trafficking and protein sorting. Int. Rev. Cytol.261, 47–116 (2007). - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- P41 GM103311/GM/NIGMS NIH HHS/United States
- R01 GM129325/GM/NIGMS NIH HHS/United States
- PJT-168907/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
- AWD-007855/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
LinkOut - more resources
Full Text Sources

