Sulfide-rich continental roots at cratonic margins formed by carbonated melts
- PMID: 39779858
- PMCID: PMC11735387
- DOI: 10.1038/s41586-024-08316-w
Sulfide-rich continental roots at cratonic margins formed by carbonated melts
Erratum in
-
Author Correction: Sulfide-rich continental roots at cratonic margins formed by carbonated melts.Nature. 2025 May;641(8061):E1. doi: 10.1038/s41586-025-08911-5. Nature. 2025. PMID: 40216944 Free PMC article. No abstract available.
Abstract
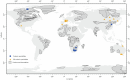
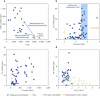
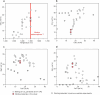
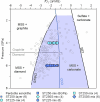
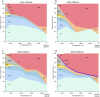
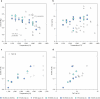
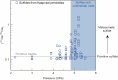
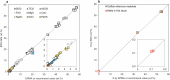
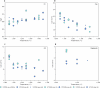
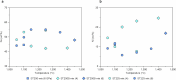
The cratonic crust contains abundant mineral deposits of metals such as gold, copper and rare earths1-5 and is underlain by a thick mantle lithosphere rich in the volatiles carbon, sulfur and water6-8. Although volatiles are known to be key components in metallogenesis9, how and where they are distributed in the cratonic lithosphere mantle and their role in the initial enrichment of metals have not been sufficiently explored. Here we compile sulfur and copper contents of global cratonic peridotites, identifying sulfide-rich and copper-rich continental roots at depths of 160-190 km at cratonic margins. Our new high-pressure experiments show that carbonated silicate melts originating from the asthenosphere lose silicate components during reaction with lithospheric peridotite, evolving to carbonatite melts that become concentrated at cratonic margins. Sulfur solubility in melts substantially decreases as the SiO2 content of melts decreases during this process, forcing sulfide precipitation and the formation of sulfide-rich continental roots at the base of the mantle lithosphere. The migration of carbonated melts towards cratonic margins replenishes the continental roots there with sulfur, explaining the co-location of magmatic metal deposits with carbonatites close to cratonic margins. These findings highlight the notable role of carbonated melts in metallogenesis and provide a potential platform for metal ore exploration.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures








References
-
- Griffin, W. L., Begg, G. C. & O’Reilly, S. Y. Continental-root control on the genesis of magmatic ore deposits. Nat. Geosci.6, 905–910 (2013). - DOI
-
- Hoggard, M. J. et al. Global distribution of sediment-hosted metals controlled by craton edge stability. Nat. Geosci.13, 504–510 (2020). - DOI
-
- Begg, G. C. et al. Lithospheric, cratonic, and geodynamic setting of Ni-Cu-PGE sulfide deposits. Econ. Geol.105, 1057–1070 (2010). - DOI
-
- Maier, W. D. & Groves, D. I. Temporal and spatial controls on the formation of magmatic PGE and Ni–Cu deposits. Miner. Depos.46, 841–857 (2011). - DOI
LinkOut - more resources
Full Text Sources

