The sequence-structure-function relationship of intrinsic ERα disorder
- PMID: 39779860
- PMCID: PMC11864982
- DOI: 10.1038/s41586-024-08400-1
The sequence-structure-function relationship of intrinsic ERα disorder
Abstract
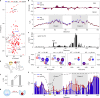
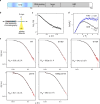
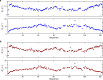
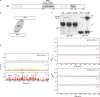
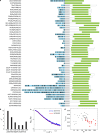
The oestrogen receptor (ER or ERα), a nuclear hormone receptor that drives most breast cancer1, is commonly activated by phosphorylation at serine 118 within its intrinsically disordered N-terminal transactivation domain2,3. Although this modification enables oestrogen-independent ER function, its mechanism has remained unclear despite ongoing clinical trials of kinase inhibitors targeting this region4-6. By integration of small-angle X-ray scattering and nuclear magnetic resonance spectroscopy with functional studies, we show that serine 118 phosphorylation triggers an unexpected expansion of the disordered domain and disrupts specific hydrophobic clustering between two aromatic-rich regions. Mutations mimicking this disruption rescue ER transcriptional activity, target-gene expression and cell growth impaired by a phosphorylation-deficient S118A mutation. These findings, driven by hydrophobic interactions, extend beyond electrostatic models and provide mechanistic insights into intrinsically disordered proteins7, with implications for other nuclear receptors8. This fundamental sequence-structure-function relationship advances our understanding of intrinsic ER disorder, crucial for developing targeted breast cancer therapeutics.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

