β-Glucan reprograms neutrophils to promote disease tolerance against influenza A virus
- PMID: 39779870
- PMCID: PMC11785525
- DOI: 10.1038/s41590-024-02041-2
β-Glucan reprograms neutrophils to promote disease tolerance against influenza A virus
Erratum in
-
Author Correction: β-Glucan reprograms neutrophils to promote disease tolerance against influenza A virus.Nat Immunol. 2025 Mar;26(3):525. doi: 10.1038/s41590-025-02099-6. Nat Immunol. 2025. PMID: 39920361 Free PMC article. No abstract available.
Abstract
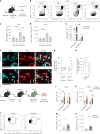
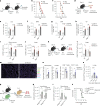
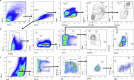
Disease tolerance is an evolutionarily conserved host defense strategy that preserves tissue integrity and physiology without affecting pathogen load. Unlike host resistance, the mechanisms underlying disease tolerance remain poorly understood. In the present study, we investigated whether an adjuvant (β-glucan) can reprogram innate immunity to provide protection against influenza A virus (IAV) infection. β-Glucan treatment reduces the morbidity and mortality against IAV infection, independent of host resistance. The enhanced survival is the result of increased recruitment of neutrophils via RoRγt+ T cells in the lung tissue. β-Glucan treatment promotes granulopoiesis in a type 1 interferon-dependent manner that leads to the generation of a unique subset of immature neutrophils utilizing a mitochondrial oxidative metabolism and producing interleukin-10. Collectively, our data indicate that β-glucan reprograms hematopoietic stem cells to generate neutrophils with a new 'regulatory' function, which is required for promoting disease tolerance and maintaining lung tissue integrity against viral infection.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures












References
-
- Divangahi, M. Are tolerance and training required to end TB? Nat. Rev. Immunol.18, 661–663 (2018). - PubMed
-
- Pernet, E., Downey, J., Vinh, D. C., Powell, W. S. & Divangahi, M. Leukotriene B4-type I interferon axis regulates macrophage-mediated disease tolerance to influenza infection. Nat. Microbiol.4, 1389–1400 (2019). - PubMed
-
- Downey, J. et al. Mitochondrial cyclophilin D promotes disease tolerance by licensing NK cell development and IL-22 production against influenza virus. Cell Rep.39, 110974 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

