Intestinal TM6SF2 protects against metabolic dysfunction-associated steatohepatitis through the gut-liver axis
- PMID: 39779889
- PMCID: PMC11774752
- DOI: 10.1038/s42255-024-01177-7
Intestinal TM6SF2 protects against metabolic dysfunction-associated steatohepatitis through the gut-liver axis
Erratum in
-
Author Correction: Intestinal TM6SF2 protects against metabolic dysfunction-associated steatohepatitis through the gut-liver axis.Nat Metab. 2025 Jan;7(1):230. doi: 10.1038/s42255-025-01215-y. Nat Metab. 2025. PMID: 39838065 Free PMC article. No abstract available.
Abstract
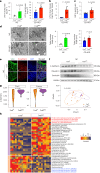
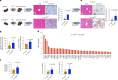
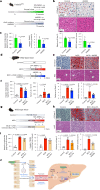
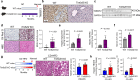
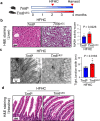
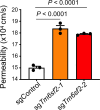
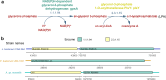
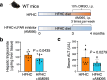
Transmembrane-6 superfamily member 2 (TM6SF2) regulates hepatic fat metabolism and is associated with metabolic dysfunction-associated steatohepatitis (MASH). TM6SF2 genetic variants are associated with steatotic liver disease. The pathogenesis of MASH involves genetic factors and gut microbiota alteration, yet the role of host-microbe interactions in MASH development remains unclear. Here, we discover that mice with intestinal epithelial cell-specific knockout of Tm6sf2 (Tm6sf2ΔIEC) develop MASH, accompanied by impaired intestinal barrier and microbial dysbiosis. Transplanting stools from Tm6sf2ΔIEC mice induces steatohepatitis in germ-free recipient mice, whereas MASH is alleviated in Tm6sf2ΔIEC mice co-housed with wild-type mice. Mechanistically, Tm6sf2-deficient intestinal cells secrete more free fatty acids by interacting with fatty acid-binding protein 5 to induce intestinal barrier dysfunction, enrichment of pathobionts, and elevation of lysophosphatidic acid (LPA) levels. LPA is translocated from the gut to the liver, contributing to lipid accumulation and inflammation. Pharmacological inhibition of the LPA receptor suppresses MASH in both Tm6sf2ΔIEC and wild-type mice. Hence, modulating microbiota or blocking the LPA receptor is a potential therapeutic strategy in TM6SF2 deficiency-induced MASH.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures










References
-
- Loomba, R., Friedman, S. L. & Shulman, G. I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell184, 2537–2564 (2021). - PubMed
-
- Yu, J., Shen, J., Sun, T. T., Zhang, X. & Wong, N. Obesity, insulin resistance, NASH and hepatocellular carcinoma. Semin. Cancer Biol.23, 483–491 (2013). - PubMed
-
- Eslam, M., Valenti, L. & Romeo, S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J. Hepatol.68, 268–279 (2018). - PubMed
MeSH terms
Substances
Grants and funding
- T12-703/19-R/Research Grants Council, University Grants Committee (RGC, UGC)
- 14117422 and 14117123/Research Grants Council, University Grants Committee (RGC, UGC)
- 82222901, 82103355 and 82272619/National Science Foundation of China | National Natural Science Foundation of China-Yunnan Joint Fund (NSFC-Yunnan Joint Fund)
- 08191336/Food and Health Bureau of the Government of the Hong Kong Special Administrative Region | Health and Medical Research Fund (HMRF)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

