High-efficiency base editing in the retina in primates and human tissues
- PMID: 39779923
- PMCID: PMC11835749
- DOI: 10.1038/s41591-024-03422-8
High-efficiency base editing in the retina in primates and human tissues
Abstract
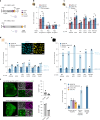
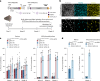
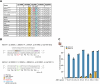
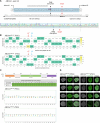
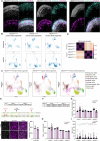
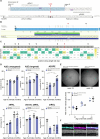
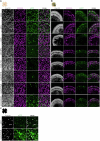
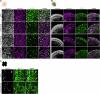
Stargardt disease is a currently untreatable, inherited neurodegenerative disease that leads to macular degeneration and blindness due to loss-of-function mutations in the ABCA4 gene. We have designed a dual adeno-associated viral vector encoding a split-intein adenine base editor to correct the most common mutation in ABCA4 (c.5882G>A, p.Gly1961Glu). We optimized ABCA4 base editing in human models, including retinal organoids, induced pluripotent stem cell-derived retinal pigment epithelial (RPE) cells, as well as adult human retinal explants and RPE/choroid explants in vitro. The resulting gene therapy vectors achieved high levels of gene correction in mutation-carrying mice and in female nonhuman primates, with average editing of 75% of cones and 87% of RPE cells in vivo, which has the potential to translate to a clinical benefit. No off-target editing was detectable in human retinal explants and RPE/choroid explants. The high editing rates in primates show promise for efficient gene editing in other ocular diseases that are targetable by base editing.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.S., C.P-W., T.V., L.A.B., L.Y., A.K., D.B. and G.C. are employees and/or shareholders of Beam Therapeutics. Beam Therapeutics has filed a patent based on some of this work. H.P.N.S. is member of the Scientific Advisory Board of: Boehringer Ingelheim, Droia NV, Janssen Research & Development (Johnson & Johnson) and Okuvision and Tenpoint Therapeutics. H.P.N.S. is a member of the Data Monitoring and Safety Board/Committee of Belite Bio (DRAGON trial, NCT05244304 ; LBS-008-CT02, NCT05266014 ), F. Hoffmann-La Roche (VELODROME trial, NCT04657289 ; DIAGRID trial, NCT05126966 ; HUTONG trial) and ViGeneron (protocol no. VG901-2021-A) and a member of the Steering Committee of Novo Nordisk (FOCUS trial; NCT03811561 ). B.R. is a consultant of SpliceBio. All other authors declare no competing interests.
Figures








References
-
- Allikmets, R. et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science277, 1805–1807 (1997). - PubMed
MeSH terms
Substances
Grants and funding
- ProgStar Study/Foundation Fighting Blindness (FFB)
- #204285/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- #PCEFP3_202756/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- #310030_192665/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- postdoctoral fellowship ALTF 688-2022/European Molecular Biology Organization (EMBO)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

