A novel prognostic risk model for patients with refractory/relapsed acute myeloid leukemia receiving venetoclax plus hypomethylating agents
- PMID: 39779979
- PMCID: PMC11879869
- DOI: 10.1038/s41375-024-02501-6
A novel prognostic risk model for patients with refractory/relapsed acute myeloid leukemia receiving venetoclax plus hypomethylating agents
Abstract
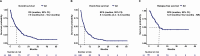
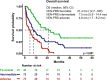
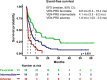
Off-label hypomethylating agents and venetoclax (HMA/VEN) are often used for relapsed and refractory (R/R) AML patients. However, predictors of outcome are elusive. The objective of the current retrospective observational multicenter study of 240 adult patients (median age 68.6 years) with R/R AML was to establish a prognostic risk score. Overall response was documented in 106 (44%) patients. With a median follow-up of 31.5 months, 179 deaths were recorded. Median overall survival (mOS) was 7.9 months. In multivariate analysis of the subgroup with molecular information (n = 174), risk factors for inferior survival included the presence of extramedullary disease, HMA pretreatment and mutations in NF1, PTPN11, FLT3, and TP53, whereas mutated SF3B1 was identified as favorable risk factor. These risk factors were subsequently applied to construct an HR-weighted risk model that allocated patients to one of three risk groups with significantly different survival outcomes: favorable (n = 46; mOS 21.4 months), intermediate (n = 75; mOS 7.5 months), and adverse (n = 53; mOS 4.6 months; p < 0.001). The model was validated in 189 AML patients treated with HMA/VEN in first line. This clinical-molecular, 3-tiered venetoclax prognostic risk score (VEN-PRS) for HMA/VEN treatment outcomes in R/R AML patients will support the selection of appropriate treatment options in this high-risk population.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: MH declares honoraria from Abbvie, Eurocept, Jazz Pharmaceuticals, Janssen, Novartis, Takeda, paid consultancy for Abbvie, Agios, BMS, Daiichi Sankyo, Glycostem, Jazz Pharmaceuticals, Kura Oncology, Novartis, Pfizer, PinotBio, Roche, Tolremo, and research funding to his institution from Abbvie, Agios, Astellas, Bayer Pharma AG, BergenBio, Daiichi Sankyo, Glycostem, Jazz Pharmaceuticals, Loxo Oncology, Novartis, Pfizer, PinotBio, Roche. RS declares support for meeting attendance from AbbVie. EK declares honoraria and/or travel support from Abbvie, JAZZ, BMS, Otsuka, Servier. WF declares honoraria from Jazz Pharmaceuticals, Pfizer, Amgen, Abbvie, Celgene, MorphoSys, Ariad/Incyte, Stemline Therapeutics, Clinigen, Daiichi Sankyo, Otsuka and Servier outside the submitted work; in addition, research support from Apis; patent issued with Amgen; support for medical writing for Amgen, Pfizer, and AbbVie. KSG declares honoraria from BMS and Abbvie. FM declares support for meeting attendance from Servier, AbbVie, Incyte, Gilead, Jazz Pharmaceuticals, Novartis, Teva, Pfizer, and Amgen, support for medical writing from Servier, and Springer Verlag, research support from Apis Technologies, and Daiichi Sankyo, honoraria from Servier, Jazz Pharmaceuticals, and AbbVie. The other authors declare no conflict of interest. Ethics approval: The registry was approved by the local Ethics Review Committee (ethical vote No.7972_BO_K_2018). Informed consent: All patients had given written informed consent to the off-label use of venetoclax, genetic analysis and use of clinical data according to the Declaration of Helsinki and institutional guidelines.
Figures
References
-
- Thol F, Döhner H, Ganser A. How I treat refractory and relapsed acute myeloid leukemia. Blood. 2024;143:11–20. - PubMed
-
- Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77. - PubMed
-
- Roloff GW, Odenike O, Bajel A, Wei AH, Foley N, Uy GL. Contemporary Approach to Acute Myeloid Leukemia Therapy in 2022. Am Soc Clin Oncol Educ Book. 2022;42:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HE 5240/6-2/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 3696/3/Deutsche Forschungsgemeinschaft (German Research Foundation)
- BE6555/1-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- BE6555/2-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 70114189/Deutsche Krebshilfe (German Cancer Aid)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

