Augmented immunogenicity of the HPV16 DNA vaccine via dual adjuvant approach: integration of CpG ODN into plasmid backbone and co-administration with IL-28B gene adjuvant
- PMID: 39780219
- PMCID: PMC11707914
- DOI: 10.1186/s12985-024-02604-7
Augmented immunogenicity of the HPV16 DNA vaccine via dual adjuvant approach: integration of CpG ODN into plasmid backbone and co-administration with IL-28B gene adjuvant
Abstract
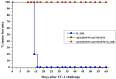
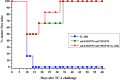
Therapeutic human papillomavirus (HPV) DNA vaccine is an attractive option to control existed HPV infection and related lesions. The two early viral oncoproteins, E6 and E7, are continuously expressed in most HPV-related pre- and cancerous cells, and are ideal targets for therapeutic vaccines. We have previously developed an HPV 16 DNA vaccine encoding a modified E7/HSP70 (mE7/HSP70) fusion protein, which demonstrated significant antitumor effects in murine models. In this study, we employed multifaceted approach to enhance the potency of the HPV16 DNA vaccine. Strategies including inserting CpG oligodeoxynucleotide (CpG ODNs) into the vaccine vector backbone, selecting cytokine gene adjuvants, combining plasmids encoding mE6/HSP70 and mE7/HSP70, and utilizing electroporation for vaccination. Our findings revealed that mice immunized with CpG-modified vaccines, coupled with an IL-28B gene adjuvant exhibited heightened antigen-specific CD8+ T cell responses. Additionally, the combination of mE6/HSP70 and mE7/HSP70 plasmids synergistically enhanced the specific CD8+ T cell response. Furthermore, vaccination with CpG-modified mE7/HSP70 and mE6/HSP70 plasmids, alongside the Interleukin-28B (IL-28B) gene adjuvant, generated substantial preventive and therapeutic antitumor effects against HPV E6- and E7-expressing tumors in C57BL/6 mice. These results suggested that integrating these multiple strategies into an HPV DNA vaccine holds promise for effectively controlling HPV infection and related diseases.
Keywords: CpG oligodeoxynucleotide; E6; E7; HPV DNA vaccine; HSP70; IL-28B gene adjuvant.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was approved by the Ethical Committee of Hebei North University (Approval No. HBNU20231222116). Consent for publication: All authors approved the publication of this manuscript. Competing interests: The authors declare no competing interests.
Figures

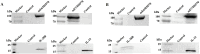



Similar articles
-
Development of DNA Vaccine Targeting E6 and E7 Proteins of Human Papillomavirus 16 (HPV16) and HPV18 for Immunotherapy in Combination with Recombinant Vaccinia Boost and PD-1 Antibody.mBio. 2021 Jan 19;12(1):e03224-20. doi: 10.1128/mBio.03224-20. mBio. 2021. PMID: 33468698 Free PMC article.
-
Enhancement of therapeutic DNA vaccine potency by melatonin through inhibiting VEGF expression and induction of antitumor immunity mediated by CD8+ T cells.Arch Virol. 2018 Mar;163(3):587-597. doi: 10.1007/s00705-017-3647-z. Epub 2017 Nov 17. Arch Virol. 2018. PMID: 29149434
-
A novel therapeutic vaccine composed of a rearranged human papillomavirus type 16 E6/E7 fusion protein and Fms-like tyrosine kinase-3 ligand induces CD8+ T cell responses and antitumor effect.Vaccine. 2017 Nov 7;35(47):6459-6467. doi: 10.1016/j.vaccine.2017.09.003. Epub 2017 Oct 10. Vaccine. 2017. PMID: 29029939
-
Genetically modified cellular vaccines for therapy of human papilloma virus type 16 (HPV 16)-associated tumours.Curr Cancer Drug Targets. 2008 May;8(3):180-6. doi: 10.2174/156800908784293596. Curr Cancer Drug Targets. 2008. PMID: 18473731 Review.
-
DNA vaccines for cervical cancer: from bench to bedside.Exp Mol Med. 2007 Dec 31;39(6):679-89. doi: 10.1038/emm.2007.74. Exp Mol Med. 2007. PMID: 18160838 Free PMC article. Review.
Cited by
-
ISG15 as a Potent Immune Adjuvant in MVA-Based Vaccines Against Zika Virus and SARS-CoV-2.Vaccines (Basel). 2025 Jun 27;13(7):696. doi: 10.3390/vaccines13070696. Vaccines (Basel). 2025. PMID: 40733673 Free PMC article.
References
-
- de Sanjosé S, Brotons M, Pavón MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2–13. PMID:28964706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

