Post-marketing safety concerns with lecanemab: a pharmacovigilance study based on the FDA Adverse Event Reporting System database
- PMID: 39780222
- PMCID: PMC11708074
- DOI: 10.1186/s13195-024-01669-4
Post-marketing safety concerns with lecanemab: a pharmacovigilance study based on the FDA Adverse Event Reporting System database
Abstract
Background: The safety data of lecanemab in the post-marketing period has yet to be fully investigated in the current literature. We aimed to identify and characterise the safety profile of lecanemab in the post-marketing period.
Methods: We searched and reviewed the reports submitted to the FDA's Adverse Event Reporting System (FAERS). We used a case/non-case approach to estimate the reporting odds ratio (ROR) and information component (IC) with 95% confidence intervals (CI) for lecanemab-related adverse events (AEs) reported at least four counts. We compared the difference between serious and non-serious reports using non-parametric tests.
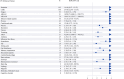
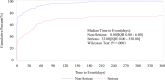
Results: The FAERS recorded 1,986 lecanemab-related AEs affecting 868 patients. Two hundred and three patients experienced serious AEs, including 22 deaths. The most frequently reported AEs were headache (n = 193), chills (n = 100), fatigue (n = 93), and amyloid-related imaging abnormality-oedema/effusion (ARIA-E) (n = 91). Safety signals were detected, such as headache (ROR: 10.4, 95%CI: 8.97, 12.07; IC: 3.25, 95%CI: 2.97, 3.40), ARIA-E (ROR: 18,299.69, 95%CI: 14,001.27, 23,917.73; IC: 13.37, 95%CI: 6.15, 6.87), and infusion-related reaction (ROR: 35.25, 95CI 27.58, 45.07; IC: 5.09, 95CI 4.15, 4.87). We also identified several new AEs, such as migraine and pancreatic carcinoma. Patients with serious AEs were more likely to be on polypharmacy for Alzheimer's disease and use aspirin, acid-suppressing medications, statins, antidepressants, or benzodiazepines compared to those with non-serious AEs.
Conclusions: Lecanemab may have a significant potential for AEs. Our results provide evidence for healthcare professionals and patients to weigh the risks and benefits of lecanemab treatment. Further prospective studies are needed to explore rare and unexpected AEs.
Keywords: Adverse event; Alzheimer’s disease; Drug safety; FAERS; Lecanemab.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval was not required as the study was conducted using de-identified publicly available data. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources

