Efficacy analysis of pancreatic duct stenting in treating severe acute pancreatitis: a retrospective study
- PMID: 39780239
- PMCID: PMC11716043
- DOI: 10.1186/s40001-024-02250-3
Efficacy analysis of pancreatic duct stenting in treating severe acute pancreatitis: a retrospective study
Abstract
Background: This study aims to evaluate the clinical efficacy of pancreatic duct stenting in the treatment of SAP, providing reference for clinical diagnosis and treatment.
Methods: A retrospective analysis was conducted on clinical data from patients with SAP admitted to the General Hospital of Ningxia Medical University from June 1, 2019 to December 31, 2022. A total of 51 patients were included (33 males, 18 females). Patients were divided into two groups based on treatment: the control group (n = 28) receiving conventional treatment and the stent group (n = 23) undergoing pancreatic duct stenting in addition to conventional treatment. Data collected and analyzed include demographic information, rates of late local complications, late surgical interventions, new-onset OF, infected pancreatic necrosis and new-onset systemic complications. Specific outcomes measured were incidences of new-onset respiratory, renal and circulatory failure, single and multiple OF, sepsis, ACS, abdominal hypertension, and pancreatogenic encephalopathy, as well as use of ≥ 3 types of antibiotics, time of antibiotic use, time of analgesic administration, oral refeeding, length of hospital stay, ICU care, and length of ICU stay. These indicators were used to assess the therapeutic efficacy of pancreatic duct stenting.
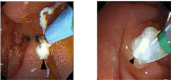
Results: All 23 patients in the stent group successfully underwent stenting. The incidence of new-onset OF and new-onset systemic complications was significantly lower in the stent group compared to the control group (χ2 = 4.96, 6.65, P < 0.05). However, no significant differences were observed between the groups regarding late local complications, infected pancreatic necrosis, and late surgical intervention (χ2 = 0.22, 0.002, 0.024, P > 0.05). Notably, two patients in the control group required additional procedures due to inadequate drainage, with one undergoing endoscopic debridement and the other, laparotomy. Mortality rates were 3 (10.7%) in the control group and 4 (17.4%) in the stent group, with no statistically significant difference (P > 0.05). Furthermore, significant differences were noted in new-onset respiratory failure, single OF, sepsis, abdominal hypertension, time of analgesic administration, oral refeeding, length of enzyme inhibitor use, and hospitalization expenses (χ2 = 3.94, 4.37, 5.79, 4.79; Z = - 2.008, - 4.176, - 4.165, - 2.309; P < 0.05). No significant differences were found in new-onset renal, circulatory, multiple OF, ACS, pancreatogenic encephalopathy, use of ≥ 3 types of antibiotics, time of antibiotic use, length of hospital stay, ICU care, and length of ICU stay (P > 0.05).
Conclusions: Pancreatic duct stenting effectively reduces the incidence of new-onset systemic complications and OF in SAP, preventing further deterioration. Pancreatic duct stenting can alleviate symptoms, shorten oral refeeding, and promote patient recovery.
Trial registration: This study was recorded as a single-center, retrospective case-control study (ChiCTR1900025833).
Keywords: Acute; Endoscopic retrograde cholangiopancreatography; Pancreatic duct stenting; Pancreatitis; Severe.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Ethics Committee of General Hospital of Ningxia Medical University (No.: KYLL-2020-16). In addition, written informed consent was obtained from all patients. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous