Myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment and their targeting in cancer therapy
- PMID: 39780248
- PMCID: PMC11707952
- DOI: 10.1186/s12943-024-02208-3
Myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment and their targeting in cancer therapy
Abstract
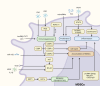
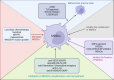
The advent of immunotherapy represents a significant breakthrough in cancer treatment, with immune checkpoint inhibitors (ICIs) targeting PD-1 and CTLA-4 demonstrating remarkable therapeutic efficacy. However, patient responses to immunotherapy vary significantly, with immunosuppression within the tumor microenvironment (TME) being a critical factor influencing this variability. Immunosuppression plays a pivotal role in regulating cancer progression, metastasis, and reducing the success rates of immunotherapy. Myeloid-derived suppressor cells (MDSCs), due to their potent immunosuppressive capabilities, emerged as major negative regulators within the TME, facilitating tumor immune evasion by modulating various immune cells. In addition to their immunosuppressive functions, MDSCs also promote tumor growth and metastasis through non-immunological mechanisms, such as angiogenesis and the formation of pre-metastatic niches. Consequently, MDSCs in the TME are key regulators of cancer immune responses and potential therapeutic targets in cancer treatment. This review describes the origins and phenotypes of MDSCs, their biological roles in tumor progression, and regulatory mechanisms, with a focus on current therapeutic approaches targeting tumor-associated MDSCs. Furthermore, the synergistic effects of targeting MDSCs in combination with immunotherapy are explored, aiming to provide new insights and directions for cancer therapy.
Keywords: Immunosuppression; Immunotherapy; MDSCs; TME; Therapeutic targets.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

