Fecal microbiota transplantation attenuates Alzheimer's disease symptoms in APP/PS1 transgenic mice via inhibition of the TLR4-MyD88-NF-κB signaling pathway-mediated inflammation
- PMID: 39780269
- PMCID: PMC11715513
- DOI: 10.1186/s12993-024-00265-8
Fecal microbiota transplantation attenuates Alzheimer's disease symptoms in APP/PS1 transgenic mice via inhibition of the TLR4-MyD88-NF-κB signaling pathway-mediated inflammation
Abstract
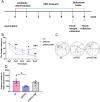
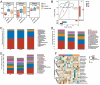
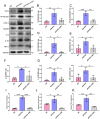
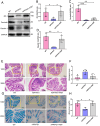
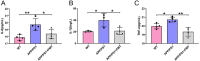
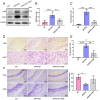
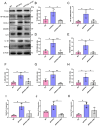
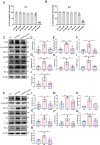
Alzheimer's disease (AD) is a prevalent and progressive neurodegenerative disorder that is the leading cause of dementia. The underlying mechanisms of AD have not yet been completely explored. Neuroinflammation, an inflammatory response mediated by certain mediators, has been exhibited to play a crucial role in the pathogenesis of AD. Additionally, disruption of the gut microbiota has been found to be associated with AD, and fecal microbiota transplantation (FMT) has emerged as a potential therapeutic approach. However, the precise mechanism of FMT in the treatment of AD remains elusive. In this study, FMT was performed by transplanting fecal microbiota from healthy wild-type mice into APP/PS1 mice (APPswe, PSEN1dE9) to assess the effectiveness of FMT in mitigating AD-associated inflammation and to reveal its precise mechanism of action. The results demonstrated that FMT treatment improved cognitive function and reduced the expression levels of inflammatory factors by regulating the TLR4/MyD88/NF-κB signaling pathway in mice, which was accompanied by the restoration of gut microbial dysbiosis. These findings suggest that FMT has the potential to ameliorate AD symptoms and delay the disease progression in APP/PS1 mice.
Keywords: Alzheimer’s disease; Fecal microbiota transplantation; Inflammation; Intestinal microbiota; Microbiota-gut-brain axis; Short-chain fatty acids.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: All animal protocols were approved by the Experimental Animal Ethics Committee of Wenzhou Medical University (Approval No. wydy2022-0375). Competing interests: The authors declare no competing interests.
Figures

References
-
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

