Safety and efficacy of immune checkpoint inhibitors in patients with pre-treatment reduced left ventricular function
- PMID: 39780280
- PMCID: PMC11707996
- DOI: 10.1186/s40959-024-00297-z
Safety and efficacy of immune checkpoint inhibitors in patients with pre-treatment reduced left ventricular function
Abstract
Aims: Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment outcomes. However, the response varies across different populations, and their use may lead to life-threatening cardiovascular (CV) events. While pre-treatment reduced left ventricular ejection fraction (LVEF) is considered a marker for high-risk cardiotoxicity and a contraindication for anthracycline and HER2-targeted therapies, there is limited evidence on the safety and efficacy of ICIs therapy in patients presenting with pre-treatment reduced LVEF. The study aims to evaluate the safety and efficacy of ICIs therapy in patients with pre-treatment reduced LVEF.
Methods: Retrospective single center cohort of patients treated with ICIs therapy, who performed pre-treatment LVEF assessment. The primary endpoint was to evaluate the safety of ICIs among this population, assessed by CV events (composite of myocarditis, acute coronary syndrome, heart failure, and arrhythmias). The secondary endpoint was to evaluate the efficacy of ICIs, assessed by all-cause mortality and progression-free survival (PFS).
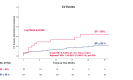
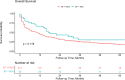
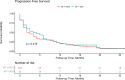
Results: The cohort included 307 patients, with 30 (10%) presenting with pre-treatment reduced LVEF, with a mean LVEF of 39 ± 7%. While a significantly higher incidence of CV events was observed in the reduced LVEF group (37% vs. 14%, p = 0.004), following a multivariate Cox regression analysis including baseline CV diseases and risk factors, pre-treatment reduced LVEF did not remain a significant independent predictor (p = 0.358). No significant differences were observed between the groups regarding all-cause mortality and PFS.
Conclusions: Pre-treatment reduced LVEF was not identified as an independent marker for clinical outcomes in patients treated with ICIs therapy.
Keywords: Cardio-oncology; Cardiotoxicity; ICIs; Immune checkpoint inhibitor; Immunotherapy; LVEF.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study was approved by the Tel Aviv Sourasky Medical Center. Helsinki Regulatory Ethics Committee (Identifier: TLV-0228-16). Consent of publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(11):1413–22. 10.1016/S1470-2045(20)30453-8. - DOI - PubMed
-
- Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344–57. 10.1016/S0140-6736(21)02098-5. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
