Dynamic Changes and Trends of SARS-CoV-2 Antibodies Induced by Infection and Vaccination Across Multiple Time Points
- PMID: 39780477
- PMCID: PMC11711922
- DOI: 10.1002/jmv.70161
Dynamic Changes and Trends of SARS-CoV-2 Antibodies Induced by Infection and Vaccination Across Multiple Time Points
Abstract
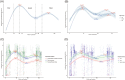
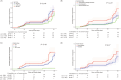
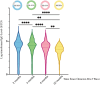
As the COVID-19 pandemic continues, increasingly complex vaccination and infection histories have made it urgent to investigate the antibody dynamics in populations with hybrid immunity. This study aimed to explore the multi-time-point dynamics of SARS-CoV-2 IgG antibody levels in a community-based population in Jiangsu Province, China, following the Omicron BA.5 wave, as well as the long-term persistence of IgG antibodies nearly 2 years postinfection. A total of 2737 participants across Jiangsu Province were followed up at three different time points over a 6-month period (December 2022-June 2023). Additionally, a cross-sectional serological survey was conducted in October 2024, involving 230 participants to assess long-term antibody persistence. We used generalized additive models to fit antibody dynamics curves, generalized linear mixed models to explore factors influencing antibody levels, and Kaplan-Meier survival analysis to estimate cumulative seroreversion rates. Our findings revealed that, following the large-scale Omicron BA.5 infections, over 85% of the population initially exhibited seropositive IgG levels. Older individuals (> 65 years) had significantly lower antibody levels and faster rates of decline compared to younger participants. Booster immunization reduced the risk of seroreversion by 59.79% (95% CI: 29.63%-76.46%), while individuals with multiple infections experienced slower antibody decay. In the cross-sectional survey conducted 22 months postinfection, the IgG seropositivity rate remained high, exceeding 98%, indicating sustained immunity at the population level. This study provides valuable insights into the dynamics and persistence of IgG antibody levels following large-scale infection. The results underscore the importance of tailored booster immunization strategies to sustain long-term immunity, especially in vulnerable groups like the elderly. Additionally, ongoing serological monitoring is essential for assessing population immunity and informing future vaccination strategies.
Keywords: SARS‐CoV‐2 IgG dynamics; booster immunization; hybrid immunity; seroepidemiology.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Carreño J. M., Alshammary H., Tcheou J., et al., “Activity of Convalescent and Vaccine Serum Against SARS‐CoV‐2 Omicron,” Nature 602, no. 7898 (2022): 682–688. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

