Efficacy and safety of telitacicept in IgA nephropathy: a real-world study
- PMID: 39780498
- PMCID: PMC11721934
- DOI: 10.1080/0886022X.2025.2449580
Efficacy and safety of telitacicept in IgA nephropathy: a real-world study
Abstract
Background: IgA nephropathy (IgAN) is the most common primary glomerular disease in the world, and specific therapeutic methods for IgAN are limited. Telitacicept is a humanized fusion protein composed of a transmembrane activator and calcium-modulating cyclophilin ligand interactor receptor and human IgG.
Aim: To evaluate the efficacy and safety of telitacicept in adult patients with IgAN in a real-world study.
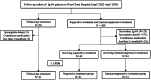
Methods: Biopsy-proven IgAN patients with 24-hour proteinuria greater than 0.5 g/d who received 240 mg telitacicept weekly were recruited for this study and 1:1:1 matched with patients who received supportive treatment only or immunosuppressive treatment by propensity score matching. The primary outcome was the change from baseline in 24-hour proteinuria over the 3-month follow-up.
Results: Twenty-one patients in each group were enrolled. Telitacicept reduced median proteinuria by 0.72 g/d (54.6%) from baseline, compared with a reduction of 0.18 g/d (20%) in the supportive treatment group and 1.12 g/d (72.1%) in the immunosuppressive treatment group. Preserved eGFR levels were observed in the telitacicept group, whereas eGFR levels decreased in the other two groups. No serious adverse events were observed in the telitacicept treatment group.
Conclusion: Telitacicept may be an effective treatment for IgAN patients by reducing proteinuria and preserving eGFR, and showed a favorable safety profile.
Keywords: BLyS/APRIL inhibitors; IgA nephropathy; kidney function; proteinuria; telitacicept.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
