Piezo1 aggravates ischemia/reperfusion-induced acute kidney injury by Ca2+-dependent calpain/HIF-1α/Notch signaling
- PMID: 39780511
- PMCID: PMC11721879
- DOI: 10.1080/0886022X.2024.2447801
Piezo1 aggravates ischemia/reperfusion-induced acute kidney injury by Ca2+-dependent calpain/HIF-1α/Notch signaling
Abstract
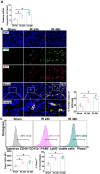
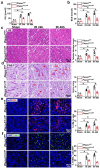
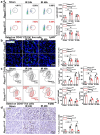
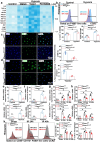
Macrophages play a vital role in the inflammation and repair processes of ischemia/reperfusion-induced acute kidney injury (IR-AKI). The mechanosensitive ion channel Piezo1 is significant in these inflammatory processes. However, the exact role of macrophage Piezo1 in IR-AKI is unknown. The main purpose of this study was to determine the role of macrophage Piezo1 in the injury and repair process in IR-AKI. Genetically modified mice with targeted knockout of Piezo1 in myeloid cells were established, and acute kidney injury was induced by bilateral renal vascular clamping surgery. Additionally, hypoxia treatment was performed on bone marrow-derived macrophages in vitro. Our data indicate that Piezo1 is upregulated in renal macrophages in mice with IR-AKI. Myeloid Piezo1 knockout provided protective effects in mice with IR-AKI. Mechanistically, the regulatory effects of Piezo1 on macrophages are at least partially linked to calpain signaling. Piezo1 activates Ca2+-dependent calpain signaling, which critically upregulates HIF-1α signaling. This key pathway subsequently influences the Notch and CCL2/CCR2 pathways, driving the polarization of M1 macrophages. In conclusion, our findings elucidate the biological functions of Piezo1 in renal macrophages, underscoring its role as a crucial mediator of acute kidney injury. Consequently, the genetic or pharmacological inhibition of Piezo1 presents a promising strategy for treating IR-AKI.
Keywords: IR-AKI; Piezo1; calpain; hypoxia; macrophage; polarization.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
