Time-resolved fluorescence anisotropies of diphenylhexatriene and perylene in solvents and lipid bilayers obtained from multifrequency phase-modulation fluorometry
- PMID: 3978080
- PMCID: PMC6952741
- DOI: 10.1021/bi00323a021
Time-resolved fluorescence anisotropies of diphenylhexatriene and perylene in solvents and lipid bilayers obtained from multifrequency phase-modulation fluorometry
Abstract
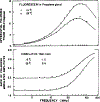
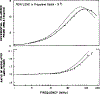
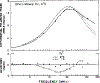
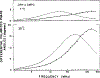
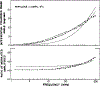
Time-resolved decays of fluorescence anisotropy were obtained from frequency-domain measurements of the phase angle difference between the parallel and perpendicular components of the polarized emission and the ratio of the modulated amplitudes. These data were measured at modulation frequencies ranging from 1 to 200 MHz. To demonstrate the general applicability of this method, we describe the resolution of both simple and complex decays of anisotropy. In particular, we resolved single, double, and triple exponential decays of anisotropy and the hindered rotational motions of fluorophores within lipid bilayers. The ease and rapidity with which these results were obtained indicate that frequency-domain measurements are both practical and reliable for the determination of complex decays of anisotropy.
Figures



References
-
- Barkley MD, Kowalczyk AA, & Brand L (1981) J.Chem. Phys 75, 3581–3593.
-
- Bevington PR (1969) Data Reduction and Error Analysis in the Physical Sciences, McGraw Hill, New York.
-
- Chen LA, Dale RE, Roth S, & Brand L (1977) J. Biol. Chem 252, 2319–2324. - PubMed
-
- Dale RE, Chen LA, & Brand L (1977) J. Biol. Chem 252, 7500–7510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
