RBT-1 reduces blood product utilization in patients undergoing nonemergency coronary artery bypass grafting and/or valve surgery
- PMID: 39780827
- PMCID: PMC11704588
- DOI: 10.1016/j.xjon.2024.06.019
RBT-1 reduces blood product utilization in patients undergoing nonemergency coronary artery bypass grafting and/or valve surgery
Conflict of interest statement
AKK is consultant for Renibus Therapeutics and consults for Medtronic, Edwards Lifesciences, Philips Research North America, Baxter, GE Healthcare, Potrero Medical, Retia Medical, and Caretaker Medical outside of this work. Drs Rodriguez, Ruiz, and Singh are employees of Renibus Therapeutics Inc. All other authors reported no conflicts of interest. The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Figures
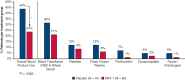
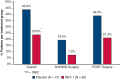

References
-
- Lamy A., Chertow G.M., Jessen M., et al. Effects of RBT-1 on preconditioning response biomarkers in patients undergoing coronary artery bypass graft or heart valve surgery: a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. EClinicalMedicine. 2024;68 doi: 10.1016/j.eclinm.2023.102364. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

