Vascular injury in glomerulopathies: the role of the endothelium
- PMID: 39780910
- PMCID: PMC11707422
- DOI: 10.3389/fneph.2024.1396588
Vascular injury in glomerulopathies: the role of the endothelium
Abstract
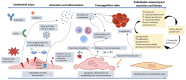
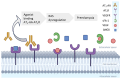
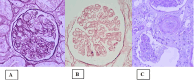
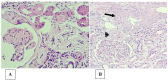
In glomerulopathies, endothelial dysfunction and the presence of histological vascular lesions such as thrombotic microangiopathy, arteriolar hyalinosis, and arteriosclerosis are related to a severe clinical course and worse renal prognosis. The endothelial cell, which naturally has anti-inflammatory and anti-thrombotic regulatory mechanisms, is particularly susceptible to damage caused by various etiologies and can become dysfunctional due to direct/indirect injury or a deficiency of protective factors. In addition, endothelial regulation and protection involve participation of the complement system, factors related to angiogenesis, the renin-angiotensin system (RAS), endothelin, the glycocalyx, the coagulation cascade, interaction between these pathways, interactions between glomerular structures (the endothelium, mesangium, podocyte, and basement membrane) and interstitial structures (tubules, arterioles and small vessels). Dysregulation of those components is also associated with the progression of renal fibrosis, since endothelial cell damage promotes endothelial-to-mesenchymal transition. Although the potential mechanisms of vascular injury have been widely described in diabetic kidney disease, hypertensive nephrosclerosis, and hemolytic uremic syndrome, they require further elucidation in other glomerulopathies. A better understanding of the pathogenesis of vascular injury in patients with glomerular diseases could contribute to the development of specific treatments for such injury.
Keywords: arteriolar hyalinosis; arteriosclerosis; glomerular endothelial cell; glomerulopathy; thrombotic microangiopathy.
Copyright © 2024 Barbosa, Câmara, Ledesma, Duarte Neto and Dias.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

