Death and survival of gut CD4 T cells following HIV-1 infection ex vivo
- PMID: 39780917
- PMCID: PMC11707799
- DOI: 10.1093/pnasnexus/pgae486
Death and survival of gut CD4 T cells following HIV-1 infection ex vivo
Abstract
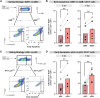
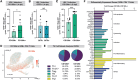
The gastrointestinal tract is ground zero for the massive and sustained CD4 T cell depletion during acute HIV-1 infection. To date, the molecular mechanisms governing this fundamental pathogenic process remain unclear. HIV-1 infection in the gastrointestinal tract is associated with chronic inflammation due to a disrupted epithelial barrier that results in microbial translocation. Here, we utilized the lamina propria aggregate culture model to demonstrate that the profound induction of granzyme B by bacteria in primary gut CD4 T cells ex vivo significantly contributes to HIV-1-mediated CD4 T cell death. Counterintuitively, a substantial fraction of gut granzyme B+ CD4 T cells harboring high levels of HIV-1 infection survive via a pathway linked to CD120b/TNFR2. Our findings underscore previously undescribed mechanisms governing the death and survival of gut CD4 T cells during HIV-1 infection that could inform strategies to counter HIV-1 pathogenesis and persistence in this critical tissue compartment.
Keywords: HIV-1; TNF response; cytotoxic CD4+ T lymphocytes; granzyme B; gut immunology.
© The Author(s) 2024. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures




References
-
- Fahey JL, et al. 1990. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 322:166–172. - PubMed
-
- Mehandru S, Dandekar S. 2008. Role of the gastrointestinal tract in establishing infection in primates and humans. Curr Opin HIV AIDS. 3:22–27. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

