Intratumoral Injection of Engineered Mycobacterium smegmatis Induces Antitumor Immunity and Inhibits Tumor Growth
- PMID: 39780958
- PMCID: PMC11704092
- DOI: 10.34133/bmr.0130
Intratumoral Injection of Engineered Mycobacterium smegmatis Induces Antitumor Immunity and Inhibits Tumor Growth
Abstract
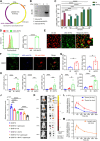
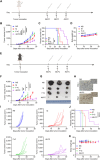
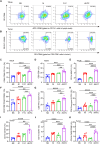
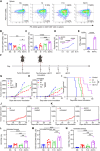
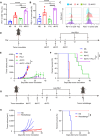
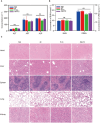
Conventional type 1 dendritic cells are essential for antigen presentation and successful initiation of antitumor CD8+ T cells. However, their abundance and function within tumors tend to be limited. Mycobacterium smegmatis, a fast-growing, nonpathogenic mycobacterium, proves to be easily modified with synthetic biology. Herein, we construct an engineered M. smegmatis expressing a fusion protein of Fms-like tyrosine kinase 3 ligand and costimulator CD40darpin (rM-FC) since the 2 drugs are reported to have a good synergistic effect. Intratumoral delivery of rM-FC effectively recruits and activates dendritic cells (DCs), especially CD103+ DCs and CD80+CD86+ DCs, further inducing sufficient migration of effector memory T cells into the tumor microenvironment. This successfully converts the so-called immune-desert tumors to the "hot" phenotype. In B16F10 mouse melanoma tumor models, local injection of rM-FC into the primary tumor triggers a robust T cell immune response to restrain the growth of both the treated tumors and the distant untreated ones. The population of PDL1+ tumor cells increased after the in situ vaccination, and murine tumors became more responsive to programmed death ligand 1 (PDL1) blockade, prompting the combination therapy. Overall, our findings demonstrate that rM-FC acts as a strong DC agonist and remarkably enhances antitumor immunity.
Copyright © 2025 Hang Zhou et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures

References
-
- Mathis T, Jardel P, Loria O, Delaunay B, Nguyen A-M, Lanza F, Mosci C, Caujolle J-P, Kodjikian L, Thariat J. New concepts in the diagnosis and management of choroidal metastases. Prog Retin Eye Res. 2019;68:144–176. - PubMed
-
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218. - PubMed
-
- Tan S, Li D, Zhu X. Cancer immunotherapy: Pros, cons and beyond. Biomed Pharmacother. 2020;124: Article 109821. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

