Functionalized Periosteum-Derived Microsphere-Hydrogel with Sequential Release of E7 Short Peptide/miR217 for Large Bone Defect Repairing
- PMID: 39780960
- PMCID: PMC11704090
- DOI: 10.34133/bmr.0127
Functionalized Periosteum-Derived Microsphere-Hydrogel with Sequential Release of E7 Short Peptide/miR217 for Large Bone Defect Repairing
Abstract
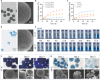
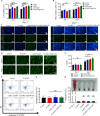
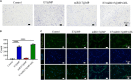
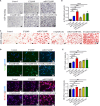
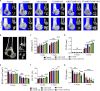
Large bone defects are still a persistent challenge in orthopedics. The availability limitations and associated complications of autologous and allogeneic bone have prompted an increasing reliance on tissue engineering and regenerative medicine. In this study, we developed an injectable scaffold combining an acellular extracellular periosteal matrix hydrogel with poly(d,l-lactate-co-glycol-acetate) microspheres loaded with the E7 peptide and miR217 (miR217/E7@MP-GEL). Characterization of the composites included morphological analysis by scanning electron microscopy, degradation and swelling tests, in vitro and in vivo biological evaluation, and the biological activity evaluation of mesenchymal stem cells (MSCs) through their effects on cell recruitment, proliferation, and osteogenic differentiation. The designed hydrogels demonstrated good physical and chemical properties that are cytocompatible and suitable for cell recruitment. In vitro studies confirmed the high biological activity of the release agent, which markedly enhanced the proliferation and osteogenic differentiation of MSCs. In vivo application to a rat model of a femur defect exhibited a significant increase in bone volume and density over 7 weeks, resulting in enhanced bone regeneration. Acellular periosteum-based hydrogels combined with the E7 peptide and miR217-loaded poly(d,l-lactate-co-glycol-acetate) microspheres can promote effective bone regeneration through the recruitment, proliferation, and osteogenic differentiation of MSCs, which provides a promising approach for the treatment of large bone defects.
Copyright © 2025 Jun Yao et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures




References
-
- Wang J, Yin Q, Gu S, Wu Y, Rui Y. Induced membrane technique in the treatment of infectious bone defect: A clinical analysis. Orthop Traumatol Surg Res. 2019;105(3):535–539. - PubMed
-
- Iseki T, Rothrauff BB, Kihara S, Overholt KJ, Taha T, Lin H, Alexander PG, Tuan RS. Enhanced osteochondral repair by leukocyte-depleted platelet-rich plasma in combination with adipose-derived mesenchymal stromal cells encapsulated in a three-dimensional photocrosslinked injectable hydrogel in a rabbit model. Stem Cell Res Ther. 2024;15(1): Article 159. - PMC - PubMed
-
- Urban IA, Montero E, Monje A, Sanz-Sánchez I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J Clin Periodontol. 2019;46(Suppl 21):319–339. - PubMed
LinkOut - more resources
Full Text Sources

