Unexpected stability of the iron(II) complex by an asymmetrical Schiff base from Fe(III): structure, magnetic and Mössbauer investigations
- PMID: 39780966
- PMCID: PMC11706641
- DOI: 10.1098/rsos.241334
Unexpected stability of the iron(II) complex by an asymmetrical Schiff base from Fe(III): structure, magnetic and Mössbauer investigations
Abstract
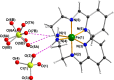
The asymmetric Schiff base prepared in situ from ethylenediamine and pyridine-2-carboxaldehyde reacts with Fe(ClO4)3·6H2O to form the Fe(II) complex [FeL2](ClO4)2 with L = N,N-diethyl-N'-(pyridin-2-yl)methylene)ethane-1,2-diamine, where the Fe(III) starting material has been unexpectedly reduced to Fe(II). This complex was characterized by elemental analysis, infrared spectra, single crystal and powder X-ray diffraction measurements, variable temperature DC magnetic measurement and room temperature Mössbauer spectroscopy. The asymmetric ligand L coordinates in a tridentate fashion through its pyridyl, azomethine and amino nitrogen atoms, generating a distorted octahedral geometry around the central metal ion. Variable temperature magnetic studies and a Mössbauer measurement show that the iron is locked in the low spin Fe(II) states.
Keywords: Mössbauer spectroscopy; asymmetrical Schiff base; low spin Fe(II) complex.
© 2025 The Author(s).
Conflict of interest statement
We declare we have no competing interests.
Figures

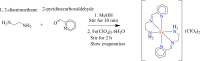

2.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/db54/11706641/82f2e0e8279f/rsos.241334.f003.gif)
References
-
- Ghamari Kargar P, Ravanjamjah A, Bagherzade G. 2021. A novel water-dispersible and magnetically recyclable nickel nanoparticles for the one-pot reduction-Schiff base condensation of nitroarenes in pure water. J. Chin. Chem. Soc. 68, 1916–1933. ( 10.1002/jccs.202100172) - DOI
-
- Naiya S, Biswas C, Drew MGB, Gómez-García CJ, Clemente-Juan JM, Ghosh A. 2010. A unique example of structural and magnetic diversity in four interconvertible copper(II)-azide complexes with the same Schiff base ligand: a monomer, a dimer, a chain, and a layer. Inorg. Chem. 49, 6616–6627. ( 10.1021/ic1005456) - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
