Drought induced metabolic shifts and water loss mechanisms in canola: role of cysteine, phenylalanine and aspartic acid
- PMID: 39781188
- PMCID: PMC11707614
- DOI: 10.3389/fpls.2024.1385414
Drought induced metabolic shifts and water loss mechanisms in canola: role of cysteine, phenylalanine and aspartic acid
Abstract
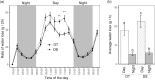
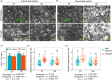
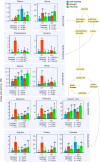
Drought conditions severely curtail the ability of plants to accumulate biomass due to the closure of stomata and the decrease of photosynthetic assimilation rate. Additionally, there is a shift in the plant's metabolic processes toward the production of metabolites that offer protection and aid in osmoadaptation, as opposed to those required for development and growth. To limit water loss via non-stomatal transpiration, plants adjust the load and composition of cuticle waxes, which act as an additional barrier. This study investigates the impact of soil water deficit on stomatal and epicuticular water losses, as well as metabolic adjustments in two canola (Brassica napus L.) cultivars-one drought-tolerant and the other drought-sensitive. Specifically, we examined the effect of a drought treatment, which involved reducing water holding capacity to 40%, on the levels of cysteine, sucrose, and abscisic acid (ABA) in the leaves of both cultivars. Next, we looked for potential differences in night, predawn, and early morning transpiration rates and the epicuticular wax load and composition in response to drought. A substantial rise in leaf cysteine was observed in both canola cultivars in response to drought, and a strong correlation was found between cysteine, ABA, and stomatal conductance, indicating that cysteine and sulfur may play a role in controlling stomatal movement during drought stress. Attributes related to CO2 diffusion (stomatal and mesophyll conductance) and photosynthetic capacity were different between the two canola cultivars suggesting a better management of water relations under stress by the drought-tolerant cultivar. Epicuticular waxes were found to adjust in response to drought, acting as an additional barrier against water loss. Surprisingly, both canola cultivars responded similarly to the metabolites (cysteine, sucrose, and ABA) and epicuticular waxes, indicating that they were not reliable stress markers in our test setup. However, the higher level of phenylalanine in the drought-tolerant canola cultivar is suggestive that this amino acid is important for adaptation to drier climates. Furthermore, a multitrait genotype-ideotype distance index (MGIDI) revealed the likely role of aspartic acid in sustaining nitrogen and carbon for immediate photosynthetic resumption after drought episodes. In conclusion, leveraging amino acid knowledge in agriculture can enhance crop yield and bolster resistance to environmental challenges.
Keywords: ABA; Brassica napus; amino acids; aspartic acid; cysteine; drought; epicuticular waxes; phenylalanine.
Copyright © 2024 Elferjani, Pahari, Soolanayakanahally, Ballantyne and Nambara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
LinkOut - more resources
Full Text Sources

