Zilebesiran and Hypertension: A Systematic Review and Meta-analysis
- PMID: 39781230
- PMCID: PMC11708905
- DOI: 10.37616/2212-5043.1408
Zilebesiran and Hypertension: A Systematic Review and Meta-analysis
Abstract
Objectives: Zilebesiran is an investigational RNA interference therapeutic designed to lower blood pressure by targeting the hepatic production of angiotensinogen, the most upstream precursor of the renin-angiotensin-aldosterone system. This approach aims to offer long-lasting blood pressure control with potentially fewer doses compared to traditional antihypertensive medications. The objective of this systematic review and meta-analysis was to assess the antihypertensive efficacy of zilebesiran in patients with hypertension.
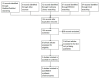
Methods: We conducted a search across PubMed, Cochrane Library, Ovid, EBSCO, up until July 2024. The eligible studies included randomized controlled trials that examined Zilebesiran versus placebo in hypertensive patients. These studies reported outcomes like reduction in 24-hour systolic blood pressure (SBP) from baseline, changes in plasma angiotensinogen (ATG) levels and office SBP at three months. Meta-analyses were carried out using RevMan.
Results: Our search identified 138 records, of which three randomized controlled trials (RCTs) with 1145 patients met inclusion criteria, focusing on Zilebesiran versus placebo for primary hypertension. Quality assessment revealed two high-quality and one moderate-quality study. Pooled analysis showed Zilebesiran significantly reduced 24-hour systolic blood pressure (SBP) compared to placebo across all doses (MD -12.84, 95% CI -16.00 to -9.68, P < 0.00001), though heterogeneity was high for doses above 500 mg. Zilebesiran also significantly lowered plasma angiotensinogen and office SBP. Sensitivity analysis resolved some heterogeneity issues. Publication bias could not be assessed.
Conclusion: Zilebesiran effectively reduces 24-hour and office systolic blood pressure and plasma angiotensinogen, demonstrating significant antihypertensive benefits. Optimal dosing appears between 250 and 500 mg. Further research should explore patient-specific responses to enhance therapeutic efficacy and minimize side effects.
Keywords: ALN-AGT01; Hypertension; Zilebesiran.
© 2024 Saudi Heart Association.
Conflict of interest statement
Conflict of interest: The authors of this study declare no conflict of interest.
Figures




References
-
- A global brief on hypertension: silent killer, global public health crisis: World Health Day 2013. n.d. [accessed August 23, 2024]. https://www.who.int/publications/i/item/a-global-brief-on-hypertension-s... .
-
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–80. doi: 10.1016/S0140-6736(21)01330-1. - DOI - PMC - PubMed
-
- Hypertension. n.d. [accessed August 23, 2024]. https://www.who.int/news-room/fact-sheets/detail/hypertension .
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
