HIF-1α Mediated Regulation of Glioblastoma Malignant Phenotypes through CD47 Protein: Understanding Functions and Mechanisms
- PMID: 39781344
- PMCID: PMC11705044
- DOI: 10.7150/jca.101050
HIF-1α Mediated Regulation of Glioblastoma Malignant Phenotypes through CD47 Protein: Understanding Functions and Mechanisms
Abstract
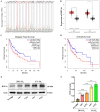
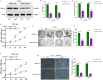
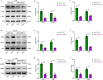
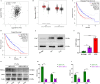
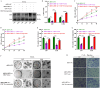
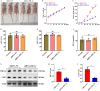
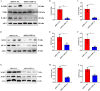
Glioblastoma (GBM) is a highly invasive and malignant primary intracranial tumor originating from glial cells, and it is associated with an extremely poor clinical prognosis. The hypoxic conditions within GBM promote various tumor cell processes such as angiogenesis, proliferation, migration, invasion, and drug resistance. A key aspect of tumor adaptation to the hypoxic environment and the promotion of malignant behaviors is the regulation of HIF-1α signaling pathways. However, the specific pathogenic mechanisms involving HIF-1α in GBM have not been thoroughly investigated. This study reveals significant overexpression of both HIF-1α and CD47 in GBM. Patients with high HIF-1α levels and CD47 expression had significantly reduced overall survival and disease-free survival times. Furthermore, a positive correlation was observed between the expression levels of HIF-1α and CD47 in GBM. Lentivirus-mediated knockdown of HIF-1α protein and plasmid-based overexpression of CD47 protein simultaneously enhanced cell proliferation, clonogenic potential and cell migration abilities in GBM, and HIF-1α was found to regulate key pathways, including the P-PI3K/P-AKT, SOX2/OCT4 and MMP2/MMP9 pathways, in GBM.
Keywords: CD47; HIF-1α; glioblastoma; migration; proliferation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
FAT1 modulates EMT and stemness genes expression in hypoxic glioblastoma.Int J Cancer. 2018 Feb 15;142(4):805-812. doi: 10.1002/ijc.31092. Epub 2017 Oct 17. Int J Cancer. 2018. PMID: 28994107
-
IDH1-R132H Suppresses Glioblastoma Malignancy through FAT1-ROS-HIF-1α Signaling.Neurol India. 2020 Sep-Oct;68(5):1050-1058. doi: 10.4103/0028-3886.294557. Neurol India. 2020. PMID: 33109851
-
Atypical induction of HIF-1α expression by pericellular Notch1 signaling suffices for the malignancy of glioblastoma multiforme cells.Cell Mol Life Sci. 2022 Oct 2;79(10):537. doi: 10.1007/s00018-022-04529-2. Cell Mol Life Sci. 2022. PMID: 36183290 Free PMC article.
-
Advances in the targeting of HIF-1α and future therapeutic strategies for glioblastoma multiforme (Review).Oncol Rep. 2017 Feb;37(2):657-670. doi: 10.3892/or.2016.5309. Epub 2016 Dec 9. Oncol Rep. 2017. PMID: 27959421 Review.
-
HIF-1α signaling: Essential roles in tumorigenesis and implications in targeted therapies.Genes Dis. 2023 Mar 30;11(1):234-251. doi: 10.1016/j.gendis.2023.02.039. eCollection 2024 Jan. Genes Dis. 2023. PMID: 37588219 Free PMC article. Review.
References
-
- Yu L, Shueng P, Chiu H. et al. Glucose Transporter 1-Mediated Transcytosis of Glucosamine-Labeled Liposomal Ceramide Targets Hypoxia Niches and Cancer Stem Cells to Enhance Therapeutic Efficacy. ACS Nano. 2023 Jul 25;17(14):13158–13175. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

