Calculation of the Minimal Clinically Important Difference in Upper and Lower Limb Motor Assessment in Spinal Muscular Atrophy
- PMID: 39781427
- PMCID: PMC11704608
- DOI: 10.2490/prm.20250001
Calculation of the Minimal Clinically Important Difference in Upper and Lower Limb Motor Assessment in Spinal Muscular Atrophy
Abstract
Objectives: Physical function assessments in patients with spinal muscular atrophy (SMA) are important indicators for assessing the effectiveness of treatment and changes over time in rehabilitation therapy. However, few reports exist on this indicator. This study calculated the minimal clinically important difference (MCID) for assessing motor function in the upper and lower limbs of individuals with SMA to estimate the degree of change within a functional score that is considered clinically meaningful.
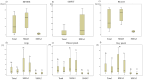
Methods: This cohort study relied on individual participant measurements. A distribution-based approach was used to calculate the MCID values, incorporating data from 26 patients with SMA for the 6-Minute Walk Test (6MWT), Hammersmith Functional Motor Scale Expanded (HFMSE), Revised Upper Limb Module (RULM), and grip and pinch strength.
Results: The standard errors of measurement for all patients were: 58.38 m for 6MWT; 4.71 points for HFMSE; 3.25 points for RULM; 10.93 N and 9.86 N for right and left grip strength, respectively; 5.42 N and 4.73 N for right and left Palmar pinch; and 11.96 N and 8.66 N for right and left Key pinch. Significant correlations were observed between the physical function assessments.
Conclusions: We calculated MCID values for physical function evaluations of SMA and, as a sub-analysis, determined the SMA type and ambulatory status. These findings are expected to contribute to future SMA treatment and rehabilitation and promote the selection of appropriate physical function assessments.
Keywords: 6-minute walk test; minimal clinically important difference; physical function assessment; spinal muscular atrophy.
2025 The Japanese Association of Rehabilitation Medicine.
Conflict of interest statement
CONFLICTS OF INTEREST: HK has received research grants from Chugai Pharmaceuticals and lecture fees from Chugai Pharmaceuticals and Biogen (Japan). The remaining authors declare no conflict of interest.
Figures

References
-
- Sugarman EA,Nagan N,Zhu H,Akmaev VR,Zhou Z,Rohlfs EM,Flynn K,Hendrickson BC,Scholl T,Sirko-Osadsa DA,Allitto BA: Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72 400 specimens. Eur J Hum Genet 2012;20:27–32. 10.1038/ejhg.2011.134 - DOI - PMC - PubMed

