The Elusive Ternary Intermediates of Chiral Phosphoric Acids in Ion Pair Catalysis─Structures, Conformations, and Aggregation
- PMID: 39782007
- PMCID: PMC11760147
- DOI: 10.1021/jacs.4c14096
The Elusive Ternary Intermediates of Chiral Phosphoric Acids in Ion Pair Catalysis─Structures, Conformations, and Aggregation
Abstract
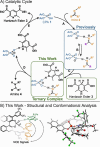
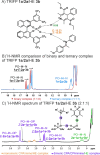
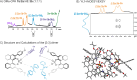
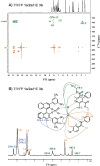
In ion-pair catalysis, the last intermediate structures prior to the stereoselective transition states are of special importance for predictive models due to the high isomerization barrier between E- and Z-substrate double bonds connecting ground and transition state energies. However, in prior experimental investigations of chiral phosphoric acids (CPA) solely the early intermediates could be investigated while the key intermediate remained elusive. In this study, the first experimental structural and conformational insights into ternary complexes with CPAs are presented using a special combination of low temperature and relaxation optimized 15N HSQC-NOESY NMR spectroscopy to enhance sensitivity. Combined NMR investigations and theoretical calculations revealed three conformers of the ternary complex, of which one also closely resembles the previously calculated transition states. In addition, a 2:1:1 ternary complex as well as an unprecedent [3:3] dimeric species consisting of two ternary complexes was revealed. Given the importance of the ground state energies for the transition state interpretation in ion pair catalysis we believe that the presented experimental insight into the structural and conformational variety of the ternary complexes is a key to the future development of predictive models in ion pair catalysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Ternary complexes of chiral disulfonimides in transfer-hydrogenation of imines: the relevance of late intermediates in ion pair catalysis.Chem Sci. 2021 Nov 17;12(46):15263-15272. doi: 10.1039/d1sc03724b. eCollection 2021 Dec 1. Chem Sci. 2021. PMID: 34976346 Free PMC article.
-
Enamine/Dienamine and Brønsted Acid Catalysis: Elusive Intermediates, Reaction Mechanisms, and Stereoinduction Modes Based on in Situ NMR Spectroscopy and Computational Studies.Acc Chem Res. 2017 Dec 19;50(12):2936-2948. doi: 10.1021/acs.accounts.7b00320. Epub 2017 Nov 27. Acc Chem Res. 2017. PMID: 29172479 Free PMC article.
-
Brønsted acid catalysis - the effect of 3,3'-substituents on the structural space and the stabilization of imine/phosphoric acid complexes.Chem Sci. 2019 Apr 8;10(20):5226-5234. doi: 10.1039/c9sc01044k. eCollection 2019 May 28. Chem Sci. 2019. PMID: 31191877 Free PMC article.
-
Recent Advances in First-Row Transition Metal/Chiral Phosphoric Acid Combined Catalysis.Top Curr Chem (Cham). 2019 Aug 28;377(5):23. doi: 10.1007/s41061-019-0249-0. Top Curr Chem (Cham). 2019. PMID: 31463700 Review.
-
Probing invisible, low-populated States of protein molecules by relaxation dispersion NMR spectroscopy: an application to protein folding.Acc Chem Res. 2008 Mar;41(3):442-51. doi: 10.1021/ar700189y. Epub 2008 Feb 15. Acc Chem Res. 2008. PMID: 18275162 Review.
Cited by
-
Development of CPA-Catalyzed β-Selective Reductive Amination of Cardenolides for the Synthesis and Biological Evaluation of Hydrolytically Stable Analogs.Chemistry. 2025 Jul 22;31(41):e202501552. doi: 10.1002/chem.202501552. Epub 2025 Jun 27. Chemistry. 2025. PMID: 40532177 Free PMC article.
References
LinkOut - more resources
Full Text Sources

