Biomimetic peptide conjugates as emerging strategies for controlled release from protein-based materials
- PMID: 39782014
- PMCID: PMC11721625
- DOI: 10.1080/10717544.2025.2449703
Biomimetic peptide conjugates as emerging strategies for controlled release from protein-based materials
Abstract
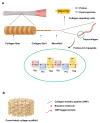
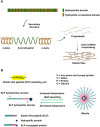
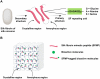
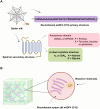
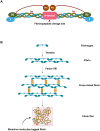
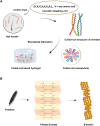
Biopolymers, such as collagens, elastin, silk fibroin, spider silk, fibrin, keratin, and resilin have gained significant interest for their potential biomedical applications due to their biocompatibility, biodegradability, and mechanical properties. This review focuses on the design and integration of biomimetic peptides into these biopolymer platforms to control the release of bioactive molecules, thereby enhancing their functionality for drug delivery, tissue engineering, and regenerative medicine. Elastin-like polypeptides (ELPs) and silk fibroin repeats, for example, demonstrate how engineered peptides can mimic natural protein domains to modulate material properties and drug release profiles. Recombinant spider silk proteins, fibrin-binding peptides, collagen-mimetic peptides, and keratin-derived structures similarly illustrate the ability to engineer precise interactions and to design controlled release systems. Additionally, the use of resilin-like peptides showcases the potential for creating highly elastic and resilient biomaterials. This review highlights current achievements and future perspectives in the field, emphasizing the potential of biomimetic peptides to transform biopolymer-based biomedical applications.
Keywords: Peptide; bioconjugation; biomimetic; collagen; elastin; fibrin; keratin; resilin; silk fibroin; spider silk.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
Elastin-Like Polypeptides for Biomedical Applications.Annu Rev Biomed Eng. 2020 Jun 4;22:343-369. doi: 10.1146/annurev-bioeng-092419-061127. Epub 2020 Apr 28. Annu Rev Biomed Eng. 2020. PMID: 32343908 Review.
-
Preparation of silk fibroin carriers for controlled release.Microsc Res Tech. 2017 Mar;80(3):312-320. doi: 10.1002/jemt.22606. Epub 2015 Dec 6. Microsc Res Tech. 2017. PMID: 26638113 Review.
-
Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics.Expert Opin Drug Deliv. 2015 May;12(5):779-91. doi: 10.1517/17425247.2015.989830. Epub 2014 Dec 5. Expert Opin Drug Deliv. 2015. PMID: 25476201 Free PMC article. Review.
-
Silk protein-based hydrogels: Promising advanced materials for biomedical applications.Acta Biomater. 2016 Feb;31:17-32. doi: 10.1016/j.actbio.2015.11.034. Epub 2015 Nov 18. Acta Biomater. 2016. PMID: 26602821 Review.
-
Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering.Int J Mol Sci. 2013 Jan 14;14(1):1629-54. doi: 10.3390/ijms14011629. Int J Mol Sci. 2013. PMID: 23344060 Free PMC article. Review.
Cited by
-
Controlling Protein Immobilization over Poly(3-hydroxybutyrate) Microparticles Using Substrate Binding Domain from PHA Depolymerase.Biomacromolecules. 2025 Apr 14;26(4):2529-2539. doi: 10.1021/acs.biomac.5c00010. Epub 2025 Mar 9. Biomacromolecules. 2025. PMID: 40059311 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
