Sustained inhibition of CSF1R signaling augments antitumor immunity through inhibiting tumor-associated macrophages
- PMID: 39782686
- PMCID: PMC11721313
- DOI: 10.1172/jci.insight.178146
Sustained inhibition of CSF1R signaling augments antitumor immunity through inhibiting tumor-associated macrophages
Abstract
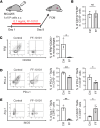
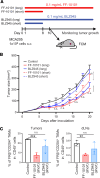
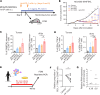
Tumor-associated macrophages (TAMs) are one of the key immunosuppressive components in the tumor microenvironment (TME) and contribute to tumor development, progression, and resistance to cancer immunotherapy. Several reagents targeting TAMs have been tested in preclinical and clinical studies, but they have had limited success. Here, we show that a unique reagent, FF-10101, exhibited a sustained inhibitory effect against colony-stimulating factor 1 receptor by forming a covalent bond and reduced immunosuppressive TAMs in the TME, which led to strong antitumor immunity. In preclinical animal models, FF-10101 treatment significantly reduced immunosuppressive TAMs and increased antitumor TAMs in the TME. In addition, tumor antigen-specific CD8+ T cells were increased; consequently, tumor growth was significantly inhibited. Moreover, combination treatment with an anti-programmed cell death 1 (anti-PD-1) antibody and FF-10101 exhibited a far stronger antitumor effect than either treatment alone. In human cancer specimens, FF-10101 treatment reduced programmed cell death 1 ligand 1 (PD-L1) expression on TAMs, as observed in animal models. Thus, FF-10101 acts as an immunomodulatory agent that can reduce immunosuppressive TAMs and augment tumor antigen-specific T cell responses, thereby generating an immunostimulatory TME. We propose that FF-10101 is a potential candidate for successful combination cancer immunotherapy with immune checkpoint inhibitors, such as PD-1/PD-L1 blockade.
Keywords: Immunology; Macrophages.
Conflict of interest statement
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

